
SUBMITTED CONFIDENTIALLY TO THE SECURITIES AND EXCHANGE COMMISSION ON October 6, 2017.
This draft registration statement has not been publicly filed with the United States Securities and Exchange Commission and all information herein remains strictly confidential.
As filed with the Securities and Exchange Commission on , 2017.
Registration No. 333-
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form S-1
REGISTRATION STATEMENT UNDER
THE SECURITIES ACT OF 1933
Motus GI Holdings, Inc.
(Exact Name of Registrant as Specified in its Charter)
| Delaware | 3841 | 81-4042793 | ||
(State or other jurisdiction of incorporation or organization) |
(Primary Standard Industrial Classification Code Number) |
(I.R.S. Employer Identification No.) |
Keren Hayesod 22
Tirat Carmel, Israel, 3902638
Telephone: 786 459 1831
(Address, including zip code, and telephone number,
including area code, of principal executive offices)
Mark Pomeranz
Chief Executive Officer
Motus GI Holdings, Inc.
1301 East Broward Boulevard, 3rd Floor
Ft. Lauderdale, FL 33301
Telephone: 786 459 1831
(Address, including zip code, and telephone number,
including area code, of agent for service)
Copies to:
Steven M. Skolnick, Esq.
Michael J. Lerner, Esq.
Lowenstein Sandler LLP
1251 Avenue of the Americas
New York, New York 10020
Telephone: (212) 262-6700
Approximate date of proposed sale to public: As soon as practicable on or after the effective date of this registration statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933 check the following box. [ ]
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. [ ]
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. [ ]
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. [ ]
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company”, and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer [ ] | Accelerated filer [ ] |
| Non-accelerated filer [ ] (Do not check if a smaller reporting company) | |
| Smaller reporting company [X] | |
| Emerging growth company [X] | |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. [X]
CALCULATION OF REGISTRATION FEE
| Title
of Each Class of Securities to Be Registered |
Proposed Maximum Aggregate Offering Price(1) | Amount of Registration Fee(2) | ||||||
| Common Stock, par value $0.001 per share | $ | $ | ||||||
| Total | $ | $ | ||||||
| (1) | Estimated solely for the purpose of calculating the registration fee in accordance with Rule 457(o) under the Securities Act of 1933. Includes the offering price of shares that the underwriters have the option to purchase to cover over-allotments, if any. |
| (2) | Calculated pursuant to Rule 457(o) based on an estimate of the proposed maximum aggregate offering price of the securities registered hereunder to be sold by the registrant. |
The Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until this Registration Statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to such Section 8(a), may determine.
The information in this preliminary prospectus is not complete and may be changed. We may not sell these securities until the Securities and Exchange Commission declares our registration statement effective. This preliminary prospectus is not an offer to sell nor does it seek an offer to buy these securities in any state where the offer or sale is not permitted.
| Preliminary Prospectus | Subject to Completion, dated October 6, 2017 |
Motus GI Holdings, Inc.

Shares
Common Stock
This is Motus GI Holdings, Inc.’s initial public offering. We are selling shares of our common stock.
We expect the public offering price to be between $ and $ per share. Currently, no public market exists for the shares. After pricing of the offering, we expect that the shares will trade on the NASDAQ Capital Market under the symbol “MOTS.”
We are an “emerging growth company” under the federal securities laws and, as such, we intend to comply with certain reduced public company reporting requirements. See “Prospectus Summary—Implications of Being an Emerging Growth Company”
Investing in our common stock involves risks that are described in the “Risk Factors” section beginning on page 6 of this prospectus.
Per Share | Total | |||||||
| Public offering price | $ | $ | ||||||
| Underwriting discount(1) | $ | $ | ||||||
| Proceeds, before expenses, to us | $ | $ |
| (1) | We refer you to “Underwriting” beginning on page 91 of this prospectus for additional information regarding total underwriter compensation. |
The underwriters may also exercise their option to purchase up to an additional shares from us at the public offering price, less the underwriting discount, for 30 days after the date of this prospectus.
The shares will be ready for delivery on or about , 2017.
Certain of our existing stockholders, including stockholders affiliated with certain of our directors, have indicated an interest in purchasing up to an aggregate of approximately $ million in shares of our common stock in this offering at the initial public offering price. However, because indications of interest are not binding agreements or commitments to purchase, the underwriters could determine to sell more, less or no shares to any of these potential investors and any of these potential investors could determine to purchase more, less or no shares in this offering.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The date of this prospectus is , 2017.
TABLE OF CONTENTS
You should rely only on the information contained in this prospectus and any related free writing prospectus that we may provide to you in connection with this offering. We have not, and the underwriters have not, authorized any other person to provide you with different information. If anyone provides you with different or inconsistent information, you should not rely on it. We are not, and the underwriters are not, making an offer to sell these securities in any jurisdiction where the offer or sale is not permitted. You should assume that the information appearing in this prospectus is accurate only as of the date on the front cover of this prospectus. Our business, financial condition, results of operations and prospects may have changed since that date.
For investors outside the United States: neither we nor any of the underwriters has done anything that would permit this offering or possession or distribution of this prospectus or any free writing prospectus we may provide to you in connection with this offering in any jurisdiction where action for that purpose is required, other than in the United States. You are required to inform yourselves about and to observe any restrictions relating to this offering and the distribution of this prospectus and any such free writing prospectus outside of the United States.
In this prospectus, we rely on and refer to information and statistics regarding our industry. We obtained this statistical, market and other industry data and forecasts from publicly available information.
This summary highlights information contained in other parts of this prospectus. Because it is a summary, it does not contain all of the information that you should consider in making your investment decision. Before investing in our common stock, you should read the entire prospectus carefully, including our consolidated financial statements and the related notes included in this prospectus and the information set forth under the headings “Risk Factors” on page 6 and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” on page 36.
When used herein, unless the context requires otherwise, references to the “Company,” “Holdings,” “we,” “our” and “us” refer to Motus GI Holdings, Inc., a Delaware corporation, collectively with our direct wholly-owned subsidiaries, Motus GI Medical Technologies, Ltd., an Israeli corporation, and Motus GI, Inc., a Delaware corporation.
All trademarks or trade names referred to in this prospectus are the property of their respective owners. Solely for convenience, the trademarks and trade names in this prospectus are referred to without the ® and ™ symbols, but such references should not be construed as any indicator that their respective owners will not assert, to the fullest extent under applicable law, their rights thereto. We do not intend the use or display of other companies’ trademarks and trade names to imply a relationship with, or endorsement or sponsorship of us by, any other companies.
Our Company
General
We have developed a single-use medical device system (the “Pure-Vu system”), cleared by the United States Food and Drug Administration (the “FDA”), that cleanses the colon during a colonoscopy procedure thereby reducing the dependency on unpleasant and time consuming pre-procedural colonoscopy preparation regimens to ensure clear visualization of the colon mucosa which is critical to procedural success. The Pure-Vu system has been designed to integrate with standard colonoscopes to enable cleaning during the procedure while preserving standard procedural workflow and techniques. We believe the Pure-Vu system has the potential to improve procedure quality, clinical outcomes and patient satisfaction while simultaneously lowering costs and improving margins for hospitals and increasing revenues and improving margins for outpatient colonoscopy clinics. Our Pure-Vu system and the procedure to cleanse the colon in preparation for colonoscopy are not currently reimbursable through private or governmental third-party payors in any country, but we do intend to seek reimbursement through private or governmental third-party payors in the future. To date, as part of our limited pilot launch, we have focused on collecting clinical data on the use of the Pure-Vu system. We do not expect to generate significant revenue from product sales unless and until we expand our commercialization efforts.
Our business was founded within the New Generation Technology (“NGT”) incubator based in Nazareth, Israel in 2008 to develop innovative endoscopy technologies, and in 2011, the business was spun out into a new company focused exclusively on the development of the Pure-Vu system. We initiated preclinical testing in 2011 and started clinical testing of the first prototype version of the Pure-Vu system in Europe in late 2012. In clinical studies performed in Europe and Israel from 2012 through the second quarter of 2017, the Pure-Vu system and earlier prototype versions have demonstrated effective cleaning in over 175 patients that followed a significantly reduced pre-procedural colonoscopy preparation regimen, as compared to current prep regimens.
Formation of Holdings
We are a Delaware corporation. In connection with our formation in September 2016, we sold an aggregate of 1,650,000 shares of our common stock for an aggregate of $82,500 ($0.05 per share), which included 450,000 shares of our common stock owned by an affiliates of Aegis Capital Corp. (“Aegis Capital”), the placement agent in our 2017 Private Placement described below.
| 1 |
The Share Exchange Transaction
Effective on December 1, 2016, Motus GI Medical Technologies Ltd., an Israeli Company (“Opco”), and the holders of all issued and outstanding shares of capital stock of Opco (the “Opco Stockholders”), entered into a share exchange agreement (the “Share Exchange Agreement”) with us. Pursuant to the terms of the Share Exchange Agreement, as a condition of and contemporaneously with the initial closing (the “Initial Closing”) of the 2017 Private Placement (defined below), the Opco Stockholders sold to us, and we acquired, all of the issued and outstanding shares of capital stock of Opco (the “Share Exchange Transaction”) and Opco became our direct wholly-owned subsidiary. Pursuant to the Share Exchange Transaction (i) the Opco Stockholders received an aggregate of 4,000,000 shares of our common stock in exchange for all of the issued and outstanding shares of capital stock of Opco, (ii) the Convertible Notes (as defined below) and the Convertible Note Warrants (as defined below) were exchanged for our securities, as described below, and (iii) all of the issued and outstanding options to purchase or otherwise acquire shares of Opco capital stock that were outstanding and unexercised as of immediately prior to the Initial Closing were substituted for 125,730 options to acquire shares of our common stock pursuant to our 2016 Equity Incentive Plan at exercise prices ranging from $2.38 to $2.52 (see “Business – Our Formation – The Share Exchange Transaction” and “Executive Compensation—2016 Equity Incentive Plan”).
Recent Developments
2017 Private Placement and Exchange of Convertible Notes
We conducted a private placement offering of units from December 2016 to February 2017 (the “2017 Private Placement”) at a purchase price of $5.00 per unit, with each unit (a “Unit”) consisting of (i) three-quarter (3/4) of a share of our common stock, and (ii) one-quarter (1/4) a share of our convertible preferred stock, par value $0.0001 (the “Series A Convertible Preferred Stock”). We issued an aggregate of 3,080,671 Units for gross proceeds of approximately $15,400,000, comprised of an aggregate of 2,310,503 shares of our common stock and 770,168 shares of Series A Convertible Preferred Stock to investors in the 2017 Private Placement, including related parties of us (see “Certain Relationships and Related Party Transactions - Convertible Note, Convertible Warrant, and 2017 Private Placement - Related Party Participation”).
In addition from June 2015 through November 2016, pursuant to the terms of a convertible note agreement, as amended (the “CNA”), Opco issued convertible notes (the “Convertible Notes”) in an aggregate amount of approximately $14.6 million (inclusive of accrued interest through December 22, 2016, the date of the initial closing of the 2017 Private Placement) to certain investors, including related parties of us and Opco (see “Certain Relationships and Related Party Transactions - Convertible Note, Convertible Warrant, and 2017 Private Placement - Related Party Participation”). As part of the 2017 Private Placement, the holders of the Convertible Notes (“Convertible Holders”) exchanged their Convertible Notes (the “Exchange of Convertible Notes”), together with accrued and unpaid interest thereon at a rate of 10% per annum, for Units in the 2017 Private Placement at a conversion price of $4.50 per Unit. In connection with the Exchange of Convertible Notes, we issued an aggregate of 3,243,744 Units, comprised of an aggregate of 2,432,808 shares of our common stock and 810,960 shares of Series A Convertible Preferred Stock. Gross proceeds from the 2017 Private Placement, inclusive of the value of the Convertible Notes, totaled approximately $30.0 million, and net proceeds to us were approximately $28.5 million.
Aegis Capital acted as the placement agent (the “Placement Agent”) for the 2017 Private Placement.
Our Risks
An investment in our common stock involves a high degree of risk. You should carefully consider the risks summarized below. These risks are discussed more fully in the “Risk Factors” section of this prospectus on page 6 herein. These risks include, but are not limited to, the following:
| ● | we have a limited operating history, our accumulated deficit as of December 31, 2016 is approximately $25.9 million, and we expect to incur substantial losses for the foreseeable future and may never achieve or maintain profitability which could materially limit our ability to raise additional funds through the issuance of new debt or equity securities or otherwise; | |
| ● | we may need to obtain additional financing to complete development and commercialization of our Pure-Vu system; |
| 2 |
| ● | the commercial success of our Pure-Vu system will depend upon its acceptance by the medical community, including physicians, patients and health care payors; | |
| ● | our Pure-Vu system and the procedure to cleanse the colon in preparation for colonoscopy are not currently reimbursable through private or governmental third-party payors in any country; | |
| ● | we are highly dependent on the success of our sole product candidate, the Pure-Vu system, which is still being commercialized; | |
| ● | we expect to rely on third parties to manufacture the components of our Pure-Vu system; | |
| ● | we currently have a limited sales and marketing organization, and in order to commercialize devices that are approved for commercial sales, we must either collaborate with third parties that have such commercial infrastructure and/or continue to develop our own sales and marketing infrastructure; | |
| ● | the manufacture of our Pure-Vu system, and the technology developed thereunder, is subject to certain Israeli government regulations which may impair our ability to outsource or transfer development or manufacturing activities with respect to any product or technology outside of Israel; | |
| ● | we face significant competition from other medical device companies; and | |
| ● | we rely on our key employees and executives and the loss of the services of our key employees and executives would adversely impact our business prospects. |
Implications of Being an Emerging Growth Company
We are an “emerging growth company,” as defined in the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”), and, for as long as we continue to be an “emerging growth company,” we may choose to take advantage of exemptions from various reporting requirements applicable to other public companies but not to “emerging growth companies,” including, but not limited to, not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, as amended, (the “Sarbanes-Oxley Act”), reduced disclosure obligations regarding executive compensation in our periodic reports, proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. We could be an “emerging growth company” for up to five years, or until the earliest of (i) the last day of the first fiscal year in which our annual gross revenues exceed $1.07 billion, (ii) the date that we become a “large accelerated filer” as defined in Rule 12b-2 under the Securities Exchange Act of 1934, as amended, which would occur if the market value of our common stock that is held by non-affiliates exceeds $700 million as of the last business day of our most recently completed second fiscal quarter, or (iii) the date on which we have issued more than $1 billion in non-convertible debt during the preceding three-year period. We intend to take advantage of these reporting exemptions described above until we are no longer an “emerging growth company.” Under the JOBS Act, “emerging growth companies” can also delay adopting new or revised accounting standards until such time as those standards apply to private companies. We have irrevocably elected not to avail ourselves of this exemption from new or revised accounting standards and, therefore, we will be subject to the same new or revised accounting standards as other public companies that are not “emerging growth companies.”
Corporate Information
We are a Delaware corporation formed in 2016 under the name Eight-Ten Merger Corp. In November 2017, we changed our name to Motus GI Holdings, Inc. We are the parent company of Motus GI Medical Technologies Ltd., an Israeli corporation, and Motus GI, Inc. a Delaware corporation.
Our principal executive offices are located at Keren Hayesod 22, Tirat Carmel, Israel, 3902638. Our web address is www.motusgi.com. Information contained in or accessible through our web site is not, and should not be deemed to be, incorporated by reference in, or considered part of, this prospectus. You should not rely on our website or any such information in making your decision whether to purchase our common stock.
“Motus GI,” “Pure-Vu,” and our other registered or common law trademarks, service marks or trade names appearing herein are the property of Motus GI Holdings, Inc. Some trademarks referred to in this prospectus are referred to without the ® and ™ symbols, but such references should not be construed as any indicator that their respective owners will not assert, to the fullest extent under applicable law, their rights thereto. We do not intend the use or display of other companies’ trademarks and trade names to imply a relationship with, or endorsement or sponsorship of us by, any other companies.
| 3 |
The following summary contains basic information about this offering. The summary is not intended to be complete. You should read the full text and more specific details contained elsewhere in this prospectus.
| Common Stock offered by us | ||
| Common Stock to be outstanding after this offering | shares | |
| Underwriters’ option to purchase additional shares from us | shares | |
| Use of Proceeds | We estimate that we will receive net proceeds from this offering of approximately $ million, or approximately $ million if the underwriters exercise their overallotment option in full, based upon an assumed initial public offering price of $ per share (the midpoint of the estimated price range set forth on the cover page of this prospectus) and after deducting the underwriting discounts and commissions and estimating offering expenses payable by us. We intend to use the net proceeds from this offering to fund the commercialization of our products and our research and development activities, as well as for working capital and other general corporate purposes. See the section entitled “Use of Proceeds” for a more complete description of the intended use of proceeds from this offering. | |
| Dividend Policy | We have never declared or paid any cash dividends on our common stock, and currently do not plan to declare cash dividends on shares of our common stock in the foreseeable future. We expect that we will retain all of our available funds and future earnings, if any, for use in the operation and expansion of our business. See “Dividend Policy” for a more complete description of our dividend policy. | |
| Risk Factors | An investment in our company is highly speculative and involves a significant degree of risk. See “Risk Factors” and other information included in this prospectus for a discussion of factors you should carefully consider before deciding to invest in shares of our common stock. |
Proposed NASDAQ Capital Market symbol “MOTS”
Certain of our existing stockholders, including stockholders affiliated with certain of our directors, have indicated an interest in purchasing up to an aggregate of approximately $ million in shares of our common stock in this offering at the initial public offering price. However, because indications of interest are not binding agreements or commitments to purchase, the underwriters could determine to sell more, less or no shares to any of these potential investors and any of these potential investors could determine to purchase more, less or no shares in this offering. Any shares purchased by our existing stockholders will be subject to lock-up restrictions described in “Shares Eligible for Future Sale.”
The number of shares of our common stock to be outstanding after this offering is based on 10,491,841 shares of common stock outstanding as of September 30, 2017 and excludes:
| ● | 1,836,844 shares of our common stock issuable upon the exercise of outstanding stock options issued under our equity incentive plan as of September 30, 2017, at exercise prices ranging from $2.38 to $4.50 per share, following the modification of share-based payment awards on September 29, 2017; |
| 4 |
| ● | 169,812 additional shares of our common stock reserved for future issuance under our 2016 Equity Incentive Plan as of September 30, 2017; | |
| ● | 1,581,128 shares of our common stock, issuable upon the conversion of Series A Convertible Preferred Stock issued in our 2017 Private Placement; | |
| ● | 907,237 shares of our common stock issuable upon the exercise of the Exchange Warrants (defined below, see “Business - Exchange of Convertible Note Warrants”), at an exercise price of $5.00 per share; | |
| ● | 403,632 shares of our common stock issuable upon the exercise of the Placement Agent Warrants (defined below, see “Business - 2017 Private Placement and Convertible Note Offering – Placement Agent Compensation”), at an exercise price of $5.00 per share; and | |
| ● | 30,000 shares of our common stock issuable upon the exercise of a consultant warrant, at an exercise price of $8.00 per share. |
Unless otherwise indicated, all information in this prospectus reflects or assumes the following:
| ● | no issuance or exercise of stock options or warrants on or after September 30, 2017; and | |
| ● | no exercise by the underwriters of their option to purchase up to an additional shares of common stock in this offering. |
| 5 |
An investment in our common stock is speculative and illiquid and involves a high degree of risk including the risk of a loss of your entire investment. You should carefully consider the following risk factors, as well as the other information in this prospectus, including our financial statements and the related notes thereto, before deciding whether to invest in shares of our common stock. These risk factors contain, in addition to historical information, forward looking statements that involve risks and uncertainties. Our actual results could differ significantly from the results discussed in the forward looking statements. The order in which the following risks are presented is not intended to reflect the magnitude of the risks described. The occurrence of any of the following adverse developments described in the following risk factors could materially and adversely harm our business, financial condition, results of operations or prospects. In such event, the value of our common stock could decline, and you could lose all or a substantial portion of the money that you pay for our common stock.
Risks Related to Our Financial Position and Need for Capital
We are a medical technology company with a limited operating history.
We are a medical technology company with a limited operating history. We received clearance from the FDA for our Pure-Vu system and have recently initiated a limited pilot launch that will run through 2018. We plan to then move into a full market launch during 2019. We expect that sales of our Pure-Vu system will account for substantially all of our revenue for the foreseeable future. However, we have limited experience in selling our products and we may be unable to successfully commercialize our Pure-Vu system for a number of reasons, including:
| ● | market acceptance of our Pure-Vu system by physicians and patients will largely depend on our ability to demonstrate its relative safety, efficacy, cost-effectiveness and ease of use; | |
| ● | our inexperience in marketing, selling and distributing our products; | |
| ● | we may not have adequate financial or other resources to successfully commercialize our Pure-Vu system; | |
| ● | we may not be able to manufacture our Pure-Vu system in commercial quantities or at an acceptable cost; | |
| ● | the uncertainties associated with establishing and qualifying a manufacturing facility; | |
| ● | patients will not generally receive reimbursement from third-party payors for the use of our Pure-Vu system for colon cleansing, which may reduce widespread use of our Pure-Vu system; | |
| ● | the introduction and market acceptance of competing products and technologies; and | |
| ● | rapid technological change may make our Pure-Vu system obsolete. |
If this offering is not successful, there is substantial doubt about our ability to continue as a going concern.
As described in Note 1 of our accompanying unaudited interim condensed consolidated financial statements, absent the proceeds of this offering or another financing, there is substantial doubt that we can continue as an ongoing business for the next twelve months. Our unaudited interim condensed consolidated financial statements do not include any adjustments that may result from the outcome of this uncertainty. If we cannot continue as a viable entity, our stockholders may lose some or all of their investment in us.
Our Pure-Vu system is currently our sole product and we are highly dependent on the successful marketing and sale of this product. There is no assurance that we will be able to develop any additional products.
Our Pure-Vu system is currently our sole product. We may fail to successfully commercialize our product. Successfully commercializing medical devices such as ours is a complex and uncertain process, dependent on the efforts of management, distributors, outside consultants, physicians and general economic conditions, among other factors. Any factors that adversely impact the commercialization of our Pure-Vu system, including, but not limited to, competition or acceptance in the marketplace, will have a negative impact on our business, results of operations and financial condition. We cannot assure you that we will be successful in developing or commercializing any potential enhancements to our Pure-Vu system or any other products. Our inability to successfully commercialize our Pure-Vu system and/or successfully develop and commercialize additional products or any enhancements to our Pure-Vu system which we may develop would have a material adverse effect on our business, results of operations and financial condition.
| 6 |
We have incurred operating losses in each year since our inception and expect to continue to incur substantial losses for the foreseeable future. We may never become profitable or, if achieved, be able to sustain profitability.
We expect to incur substantial expenses without corresponding revenues unless and until we expand our commercialization efforts. To date, as part of our limited launch, we have generated some revenue from our Pure-Vu system, but we do not expect to generate significant revenue from product sales unless and until we expand our commercialization efforts, which we expect will take a number of years and is subject to significant uncertainty. We expect to incur significant marketing expenses in the United States and elsewhere, and there can be no assurance that we will generate significant revenues or ever achieve profitability. Our net loss for the six months ended June 30, 2017 and June 30, 2016 was approximately $6.3 million and $3.7 million respectively. Our net loss for the years ended December 31, 2016 and December 31, 2015 was approximately $8.0 million and $6.0 million, respectively. As of June 30, 2017, we had an accumulated deficit of approximately $32.2 million.
Our cash or cash equivalents will only fund our operations for a limited time and we will need to raise additional capital to support our development and commercialization efforts.
We are currently operating at a loss and expect our operating costs will increase significantly as we incur costs related to the commercialization of our Pure-Vu system. At June 30, 2017, we had a cash and cash equivalents balance of approximately $12.9 million. Based on our current business plan, we believe the net proceeds from this offering, together with our current cash and cash equivalents, will be sufficient to meet our anticipated cash requirements over at least the next 18 months. If our available cash balances and net proceeds from this offering are insufficient to satisfy our liquidity requirements, including due to risks described in this prospectus, we may seek to raise additional capital through equity offerings, debt financings, collaborations or licensing arrangements. We will need to raise additional capital, and we may also consider raising additional capital in the future to expand our business, to pursue strategic investments, to take advantage of financing opportunities, or for other reasons, including to:
| ● | fund development and efforts of any future products; | |
| ● | acquire, license or invest in technologies; | |
| ● | acquire or invest in complementary businesses or assets; and | |
| ● | finance capital expenditures and general and administrative expenses. |
Our present and future funding requirements will depend on many factors, including:
| ` | ● | our revenue growth rate and ability to generate cash flows from operating activities; |
| ● | our sales and marketing and research and development activities; | |
| ● | costs of and potential delays in product development; | |
| ● | changes in regulatory oversight applicable to our products; and | |
| ● | costs related to international expansion. |
We do not currently have any arrangements or credit facilities in place as a source of funds, and there can be no assurance that we will be able to raise sufficient additional capital on acceptable terms, or at all. We may seek additional capital through a combination of private and public equity offerings, debt financings and strategic collaborations. Debt financing, if obtained, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, that could increase our expenses and require that our assets secure such debt. Equity financing, if obtained, could result in dilution to our then existing stockholders and/or require such stockholders to waive certain rights and preferences. If such financing is not available on satisfactory terms, or is not available at all, we may be required to delay, scale back or eliminate the development of business opportunities and our operations and financial condition may be materially adversely affected. We can provide no assurances that any additional sources of financing will be available to us on favorable terms, if at all. In addition, if we are unable to secure sufficient capital to fund our operations, we might have to enter into strategic collaborations that could require us to share commercial rights to the Pure-Vu system with third parties in ways that we currently do not intend or on terms that may not be favorable to us. If we choose to pursue additional indications and/or geographies for the Pure-Vu system or otherwise expand more rapidly than we presently anticipate, we may also need to raise additional capital sooner than expected.
| 7 |
Risks Related to Government Regulation and Third-Party Reimbursement
We are subject to complex and costly regulation.
Our product, and any products we may develop in the future, are subject to regulation by the FDA and other national, supranational, federal and state governmental authorities. It can be costly and time-consuming to obtain regulatory clearance and/or approval to market a new or modified medical device or other product. Clearance and/or approval might not be granted on a timely basis, if at all. Regulations are subject to change as a result of legislative, administrative or judicial action, which may further increase our costs or reduce sales. Unless an exception applies, the FDA requires that the manufacturer of a new medical device or a new indication for use of, or other significant change in, an existing medical device obtain either FDA 510(k) pre-market clearance or pre-market approval before that product can be marketed or sold in the United States. Modifications or enhancements to a product that could significantly affect its safety or effectiveness, or that would constitute a major change in the intended use of the device, technology, materials, labeling, packaging, or manufacturing process may also require a new 510(k) clearance. The FDA has indicated that it intends to continue to enhance its pre-market requirements for medical devices. Although we cannot predict with certainty the future impact of these initiatives, it appears that the time and cost to get medical devices to market could increase significantly.
In addition, we are subject to regulations that govern manufacturing practices, product labeling and advertising, and adverse-event reporting that apply after we have obtained clearance or approval to sell a product. Our failure to maintain clearance for our Pure-Vu system, to obtain clearance or approval for new or modified products, or to adhere to regulations for manufacturing, labeling, advertising or adverse event reporting could adversely affect our results of operations and financial condition. Further, if we determine a product manufactured or marketed by us does not meet our specifications, published standards or regulatory requirements, we may seek to correct the product or withdraw the product from the market, which could have an adverse effect on our business. Many of our facilities and procedures, and those of our suppliers are subject to ongoing oversight, including periodic inspection by governmental authorities. Compliance with production, safety, quality control and quality assurance regulations can be costly and time-consuming.
The sales and marketing of medical devices is under increased scrutiny by the FDA and other enforcement bodies. If our sales and marketing activities fail to comply with FDA regulations or guidelines, or other applicable laws, we may be subject to warnings or enforcement actions from the FDA or other enforcement bodies.
We may be unable to obtain or maintain governmental approvals to market our Pure-Vu system outside the United States, including the European Union countries.
Any medical device placed on the European market must comply with the relevant legislation of the European Economic Community, or EEC, which requires manufacturers of medical products to obtain the right to affix the CE Mark to their products before selling them in the European Union, the European Economic Area and Switzerland. The CE Mark is an international symbol of adherence to quality assurance standards and compliance with applicable European medical device directives. In order to obtain the right to affix the CE Mark to products, a manufacturer must obtain certification that its processes meet specified quality and design standards. Compliance with the medical device directives, as certified by an organization designated by a European Union country to assess the conformity of certain products before being placed on the market (a “Notified Body”), permits the manufacturer to affix the CE Mark on its products and commercially distribute those products throughout the European Union, the European Economic Area and Switzerland. However, individual countries can lawfully request that a medical device be registered locally. Furthermore, countries may have requirements in place in relation to the language of the device information, which would require additional compliance, review and approval. In addition, the European Union is presently assessing an overhaul of its regulatory requirements that may make the CE Mark much more difficult to obtain or maintain. We applied for a CE Mark in Europe in April 2017, however, there can be no assurance that we will be granted CE Mark, and the failure to do so could adversely impact our revenues.
| 8 |
To be able to market and sell our Pure-Vu system in other countries, we must obtain regulatory approvals and comply with the regulations of those countries. These regulations, including the requirements for approvals and the time required for regulatory review, vary from country to country. Many non-European markets, including major markets in South America and Asia Pacific, have allowed for expedited regulatory review and approval based on an existing CE Mark. We intend to target countries with a regulatory approval process with similar requirements to CE Mark. However, obtaining and maintaining foreign regulatory approvals are complex and expensive and subject to delays, and management cannot be certain that we will receive and be able to maintain regulatory approvals in any foreign country in which we plan to market our Pure-Vu system or in the time frame in which we expect.
Modifications to our product may require new 510(k) clearance or may require us to cease marketing or recall the modified products until approvals are obtained.
After a device receives 510(k) clearance, any modification that could significantly affect its safety or effectiveness, or that would constitute a major change in its intended use, will require a new clearance. Changes that do not rise to this level of significance, including certain manufacturing changes, may be made without FDA clearance upon documentation in the manufacturer’s files of the determination of the significance of the change. The FDA requires each manufacturer to make this determination initially, but the FDA can review any such decision and can disagree with the manufacturer’s determination. If the FDA disagrees with any determination that we may make in the future and requires us to seek new 510(k) clearance for modifications to any previously approved or cleared products for which we have concluded that new approvals are unnecessary, we may be required to cease marketing or distribution of our products or to recall the modified product until we obtain approval, and we may be subject to significant regulatory fines or penalties. We intend in the future to expand the indication for which the Pure-Vu system is cleared or approved to allow us to actively promote the product and a less-prep regimen to patients. We plan to perform a clinical trial that should facilitate approval of expanded labeling, however, if the FDA denies our expanded labeling our revenues will be adversely affected.
In the European Union/European Economic Area (the “EU/EEA”), we will be required to inform the Notified Body that carried out the conformity assessment of the medical devices we market or sell in the EEA of any planned changes to our quality system or changes to our devices which could affect compliance with the essential requirements set forth in the EU Medical Devices Directive or the devices’ intended purpose. The Notified Body will then assess the changes and verify whether they affect the products’ conformity with the essential requirements set forth in the EU Medical Devices Directive or the conditions for the use of the device. If the assessment is favorable, the Notified Body will issue a new CE Certificate of Conformity or an addendum to the existing CE Certificate of Conformity attesting compliance with the essential requirements set forth in the EU Medical Devices Directive. If it is not, we may not be able to market and sell the product in the EEA.
If our product malfunctions, or causes or contributes to a death or a serious injury, we will be subject to medical device reporting regulations, which can result in voluntary corrective actions or agency enforcement actions.
Under the FDA medical device reporting regulations, medical device manufacturers are required to report to the FDA information that a device has or may have caused or contributed to a death or serious injury or has malfunctioned in a way that would likely cause or contribute to death or serious injury if the malfunction of the device or one of our similar devices were to recur. If we fail to report these events to the FDA within the required timeframes, or at all, FDA could take enforcement action against us. Any such adverse event involving our product also could result in future voluntary corrective actions, such as recalls or customer notifications, or agency action, such as inspection or enforcement action. Any corrective action, whether voluntary or involuntary, as well as defending ourselves in a lawsuit, will require the dedication of our time and capital, distract management from operating our business, and may harm our reputation and financial results.
| 9 |
Our Pure-Vu system may in the future be subject to product recalls. A recall of our products, either voluntarily or at the direction of the FDA or another governmental authority, including a third-country authority, or the discovery of serious safety issues with our products, could have a significant adverse impact on us.
The FDA and similar foreign governmental authorities have the authority to require the recall of commercialized products in the event of material deficiencies or defects in design or manufacture. In this case, the FDA, the authority to require a recall must be based on an FDA finding that there is reasonable probability that the device would cause serious injury or death. In addition, foreign governmental bodies have the authority to require the recall of our products in the event of material deficiencies or defects in design or manufacture. Manufacturers may, under their own initiative, recall a product if any material deficiency in a device is found. The FDA requires that certain classifications of recalls be reported to the FDA within 10 working days after the recall is initiated. A government-mandated or voluntary recall by us or a distributor could occur as a result of an unacceptable risk to health, component failures, malfunctions, manufacturing errors, design or labeling defects or other deficiencies and issues. A recall of our products would divert managerial and financial resources and have an adverse effect on our reputation, results of operations and financial condition, which could impair our ability to produce our product in a cost-effective and timely manner in order to meet our customers’ demands. We may also be subject to liability claims, be required to bear other costs, or take other actions that may have a negative impact on our future sales and our ability to generate profits. Companies are required to maintain certain records of recalls, even if they are not reportable to the FDA or another third-country competent authority. We may initiate voluntary recalls that we determine do not require notification of the FDA or another third-country competent authority. If the FDA disagrees with our determinations, they could require us to report those actions as recalls. A future recall announcement could harm our reputation with customers and negatively affect our sales. In addition, the FDA could take enforcement action for failing to report the recalls.
We are also required to follow detailed recordkeeping requirements for all firm-initiated medical device corrections and removals. In addition, in December of 2012, the FDA issued a draft guidance intended to assist the FDA and industry in distinguishing medical device recalls from product enhancements. Per the guidance, if any change or group of changes to a device that addresses a violation of the Federal Food, Drug and Cosmetic Act (the “FDCA”), that change would generally constitute a medical device recall and require submission of a recall report to the FDA.
Our Pure-Vu system is not currently reimbursable through private or governmental third-party payors, which could limit market acceptance.
Our Pure-Vu system and the procedure to cleanse the colon in preparation for colonoscopy are not currently reimbursable through private or governmental third-party payors in any country. We do intend to seek reimbursement through private or governmental third-party payors in the future, however coverage and reimbursement may not be available for any product that we commercialize and, even if available, the level of reimbursement may not be satisfactory. The commercialization of our Pure-Vu system depends on prospective patients’ ability to cover the costs of the procedure, and/or physician willingness to subsidize all or some of the cost of the procedure in order to decrease the likelihood of a failed colonoscopy due to poor preparation and increase the number of colonoscopies performed during a typical day. We believe that a substantial portion of individuals who are candidates for the use of the Pure-Vu system worldwide do not have the financial means to cover its cost. Moreover, healthcare providers may not have confidence in the cost savings and revenue generating potential that use of the Pure-Vu system may offer, and may be reluctant to make the initial investment in the system. A general regional or worldwide economic downturn could negatively impact demand for our Pure-Vu system. In the event that medically eligible patients deem the costs of our procedure to be prohibitively high or consider alternative treatment options to be more affordable, or healthcare providers deem the economic benefits do not outweigh the cost of the system, our business, results of operations and financial condition would be negatively impacted.
If we or our sales personnel or distributors do not comply with state fraud and abuse laws, including anti-kickback laws for any products approved in the U.S., or with similar foreign laws where we market our products, we could face significant liability.
There are numerous state laws pertaining to healthcare fraud and abuse, including anti-kickback laws, false claims, and physician transparency laws. Our relationships with physicians and surgeons, hospitals and our independent distributors are subject to scrutiny under these laws. Violations of these laws are punishable by criminal and civil sanctions, including significant fines, damages and monetary penalties and in some instances, imprisonment and exclusion from participation in federal and state healthcare programs, including the Medicare, Medicaid and Veterans Administration health programs.
| 10 |
Many foreign countries have enacted similar laws addressing fraud and abuse in the healthcare sector. The shifting commercial compliance environment and the need to build and maintain robust and expandable systems to comply with different compliance requirements in multiple jurisdictions increases the possibility that a healthcare company may run afoul of one or more of the requirements.
We may become liable for significant damages or be restricted from selling our products if we engage in inappropriate promotion of our Pure-Vu system.
Our promotional materials and training methods for our Pure-Vu system must comply with FDA and other applicable laws and regulations, including the prohibition of the promotion of the “off-label” use of our Pure-Vu system, including by using our Pure-Vu system in a way not approved by the FDA. The Pure-Vu system is currently indicated to connect to standard colonoscopes to facilitate intra-procedural cleaning of a poorly prepared colon by irrigating or cleaning the colon and evacuating the irrigated fluid, feces and other bodily fluids and matter. Healthcare providers may use our products off-label, as the FDA does not restrict or regulate a physician’s choice of treatment within the practice of medicine. However, if the FDA determines that our promotional materials, training or marketing efforts constitutes promotion of an off-label use, it could request that we modify our training or promotional materials or marketing efforts or subject us to regulatory or enforcement actions, including the issuance of an untitled letter, a warning letter, injunction, seizure, civil fine and criminal penalties. It is also possible that other federal, state or foreign enforcement authorities might take action if they consider our promotional or training materials to constitute promotion of an unapproved use, which could result in significant fines or penalties. Although we do not intend to engage in any activities that may be considered off-label promotion of our products, the FDA or another regulatory agency could disagree and conclude that we have engaged, directly or indirectly, in off-label promotion. In addition, the off-label use of our products may increase the risk of product liability claims. Product liability claims are expensive to defend and could result in substantial damage awards against us and harm our reputation.
Our financial performance may be adversely affected by medical device tax provisions in the healthcare reform laws.
The Patient Protection and Affordable Care Act imposes, among other things, an excise tax of 2.3% on any entity that manufactures or imports medical devices offered for sale in the United States, although this tax has been suspended for calendar years 2016 and 2017. It is unclear at this time if the moratorium will be extended. We anticipate that primarily all of our sales of our Pure-Vu system in the United States will be subject to this 2.3% excise tax after December 31, 2017. Additionally, Congress could terminate the moratorium or further change the law related to the medical device tax in a manner that could adversely affect us.
Risks Related to Our Business Operations
The Pure-Vu system may not be accepted by physicians and patients.
Our Pure-Vu system for use during colonoscopy screenings to clean the colon through irrigation and evacuation of bowel contents is a new technology and may be perceived as more invasive than current colonoscopy screening procedures, and patients may be unwilling to undergo the procedure. Moreover, patients may be unwilling to depart from the current standard of care. In addition, physicians tend to be slow to change their medical treatment practices because of perceived liability risks arising from the use of new products. Physicians may not recommend or prescribe our Pure-Vu system until there is long-term clinical evidence to convince them to alter their existing treatment methods, there are recommendations from prominent physicians that our Pure-Vu system is safe and efficient and reimbursement or insurance coverage is available. We cannot predict when, if ever, physicians and patients may adopt the use of our Pure-Vu system. If our Pure-Vu system does not achieve an adequate level of acceptance by patients, physicians and healthcare payors, we may not generate significant product revenue and we may not become profitable.
Accordingly, you should consider our prospects in light of the costs, uncertainties, delays and difficulties frequently encountered by early commercial stage companies. Potential investors should carefully consider the risks and uncertainties that a company with a limited operating history will face. In particular, potential investors should consider that we cannot assure you that we will be able to:
| 11 |
| ● | successfully execute our current business plan for the commercialization of our Pure-Vu system, or that our business plan is sound; | |
| ● | successfully contract for and establish a commercial supply of our product; | |
| ● | achieve market acceptance of our Pure-Vu system; and | |
| ● | attract and retain an experienced management and advisory team. |
If we cannot successfully execute any one of the foregoing, our business may not succeed and your investment will be adversely affected.
If we are not able to successfully commercialize our Pure-Vu system, the revenue that we generate from its sales, if any, may be limited.
The commercial success of our Pure-Vu system will depend upon its acceptance by the medical community, including physicians, patients and health care payors. The degree of market acceptance of our Pure-Vu system will depend on a number of factors, including:
| ● | demonstration of clinical safety and efficacy; | |
| ● | relative convenience, burden and ease of administration; | |
| ● | the prevalence and severity of any adverse effects; | |
| ● | the willingness of physicians to prescribe the Pure-Vu system and of the target patient population to try new procedures; | |
| ● | efficacy of our Pure-Vu system compared to competing procedures; | |
| ● | the introduction of any new products and procedures that may in the future become available for colonoscopy preparation may be approved; | |
| ● | pricing and cost-effectiveness; | |
| ● | the inclusion or omission of our Pure-Vu system in applicable treatment guidelines; | |
| ● | the effectiveness of our or any future collaborators’ sales and marketing strategies; | |
| ● | limitations or warnings contained in FDA-approved labeling; | |
| ● | our ability to obtain and maintain sufficient third-party coverage or reimbursement from government health care programs, including Medicare and Medicaid, private health insurers and other third-party payors; and | |
| ● | the willingness of patients to pay out-of-pocket in the absence of third-party coverage or reimbursement. |
If our Pure-Vu system does not achieve an adequate level of acceptance by physicians, health care payors and patients, we may not generate sufficient revenue and we may not be able to achieve or sustain profitability. Our efforts to educate the medical community and third-party payors on the benefits of our Pure-Vu system may require significant resources and may never be successful.
We currently have a limited sales and marketing organization. If we are unable to secure a sales and marketing partner and/or establish satisfactory sales and marketing capabilities, we may not successfully commercialize our Pure-Vu system.
At present, we have limited sales or marketing personnel. In order to commercialize devices that are approved for commercial sales, we must either collaborate with third parties that have such commercial infrastructure and/or continue to develop our own sales and marketing infrastructure. If we are not successful entering into appropriate collaboration arrangements, or recruiting sales and marketing personnel or in building a sales and marketing infrastructure, we will have difficulty successfully commercializing our Pure-Vu system, which would adversely affect our business, operating results and financial condition.
We may not be able to enter into collaboration agreements on terms acceptable to us or at all. In addition, even if we enter into such relationships, we may have limited or no control over the sales, marketing and distribution activities of these third parties. Our future revenues may depend heavily on the success of the efforts of these third parties. If we elect to establish a sales and marketing infrastructure we may not realize a positive return on this investment. In addition, we will have to compete with established and well-funded medical device companies to recruit, hire, train and retain sales and marketing personnel. Factors that may inhibit our efforts to commercialize our Pure-Vu system without strategic partners or licensees include:
| 12 |
| ● | our inability to recruit and retain adequate numbers of effective sales and marketing personnel; | |
| ● | the inability of sales personnel to obtain access to or persuade adequate numbers of physicians to use our Pure-Vu system; | |
| ● | the lack of complementary products to be offered by sales personnel, which may put us at a competitive disadvantage relative to companies with more extensive product lines; and | |
| ● | unforeseen costs and expenses associated with creating an independent sales and marketing organization. |
Our Pure-Vu system may exhibit adverse side effects that prevent its widespread adoption or that may necessitate its withdrawal from the market.
Our Pure-Vu system is currently believed to have the same side effects as a standard colonoscopy, such as inducing trauma to the colon’s mucosa or in rare cases perforation of the colon. With more extensive use the Pure-Vu system may be found to cause additional undesirable and unintended side effects or show a higher rate of side effects than a standard colonoscopy that may prevent or limit its commercial adoption and use. Even upon receiving clearance from the FDA and other regulatory authorities, our products may later exhibit adverse side effects that prevent widespread use or necessitate withdrawal from the market. The manifestation of such side effects could cause our business to suffer.
If we do not convince gastroenterologists that our products are attractive alternatives to the current pre-procedure bowel preparation regimen as well as an additional source of income, we will not be commercially successful.
Gastroenterologists will play a significant role in determining the course of pre-procedure bowel preparation for colonoscopies and, ultimately, the type of products and procedures that will be used to prepare a patient for a colonoscopy. As a result, it will be important for us to effectively market our products to them. Acceptance of our products depends on educating gastroenterologists as to the distinctive characteristics, perceived clinical benefits, safety and cost effectiveness of our products as compared to the current standard of care as well as the economic benefit that an additional source of income will provide to a gastroenterological practice. It also depends on training gastroenterologists in the proper application of our products. If we are not successful in convincing gastroenterologists of the merits of our products or educating them on the use of our products, they may not use our products and we will be unable to fully commercialize our products or reach profitability. Gastroenterologists may be hesitant to change their medical treatment practices for the following reasons, among others:
| ● | lack of experience with our products and concerns regarding potential side effects; | |
| ● | lack of clinical data currently available to support the safety and effectiveness of our products; | |
| ● | lack or perceived lack of evidence supporting additional patient benefits; | |
| ● | perceived liability risks generally associated with the use of new products and procedures; and | |
| ● | the time commitment that may be required for training. |
In addition, we believe recommendations and support of our products by influential gastroenterologists are important for market acceptance and adoption. If we do not receive support from such gastroenterologists or long term data does not show the benefits of using our products, gastroenterologists may not use our products. In such circumstances, we may not be able to grow our revenues or achieve profitability.
If we are unable to train gastroenterologists and their clinical staff on the safe and appropriate use of our products, we may be unable to achieve revenue growth or profitability.
An important part of our sales process includes the ability to train gastroenterologists and their clinical staff on the safe and appropriate use of our products. We have very limited experience in training and retaining qualified independent gastroenterologists to perform the colon cleansing procedure using our Pure-Vu system. If we are unable to attract gastroenterologists to our training programs, we may be unable to achieve growth or profitability.
| 13 |
There is a learning process involved in gastroenterologists and their clinical staff becoming proficient in the use of our products. It is critical to the success of our commercialization efforts to train a sufficient number of gastroenterologists and to provide them with adequate instruction in the use of our Pure-Vu system. This training process may take longer than expected and may therefore affect our ability to increase sales. Following completion of training, we expect to rely on the trained gastroenterologists to advocate the benefits of our products in the broader marketplace. Convincing gastroenterologists to dedicate the time and energy necessary for adequate training is challenging, and we cannot assure you we will be successful in these efforts. If gastroenterologists and their clinical staff are not properly trained, they may misuse or ineffectively use our products. Such uses may result in unsatisfactory patient outcomes, patient injury, negative publicity or lawsuits against us, any of which would have a material adverse effect on our business, results of operations and financial condition.
We may face competition from other medical device companies in the future and our operating results will suffer if we fail to compete effectively.
The medical device industries are intensely competitive and subject to rapidly evolving technology and intense research and development efforts. We have competitors in a number of jurisdictions that have substantially greater name recognition, commercial infrastructures and financial, technical and personnel resources than we have. Established competitors may invest heavily to quickly discover and develop novel devices or procedures that could make our Pure-Vu system obsolete or uneconomical. Any new product that competes with a cleared medical device may need to demonstrate compelling advantages in efficacy, cost, convenience, tolerability and safety to be commercially successful. Other competitive factors could force us to lower prices or could result in reduced sales. In addition, new devices developed by others could emerge as competitors to our Pure-Vu system. If we are not able to compete effectively against our current and future competitors, our business will not grow and our financial condition and operations will suffer.
Our future growth depends, in part, on our ability to penetrate foreign markets, where we would be subject to additional regulatory burdens and other risks and uncertainties.
Our future profitability will depend, in part, on our ability to commercialize our Pure-Vu system in foreign markets for which we intend to rely on collaborations with third parties. If we commercialize our Pure-Vu system in foreign markets, we would be subject to additional risks and uncertainties, including:
| ● | our customers’ ability to obtain reimbursement for our Pure-Vu system in foreign markets; | |
| ● | our inability to directly control commercial activities because we are relying on third parties; | |
| ● | the burden of complying with complex and changing foreign regulatory, tax, accounting and legal requirements; | |
| ● | different medical practices and customs in foreign countries affecting acceptance in the marketplace; | |
| ● | import or export licensing requirements; | |
| ● | longer accounts receivable collection times; | |
| ● | longer lead times for shipping; | |
| ● | language barriers for technical training; | |
| ● | reduced protection of intellectual property rights in some foreign countries; | |
| ● | foreign currency exchange rate fluctuations; and | |
| ● | the interpretation of contractual provisions governed by foreign laws in the event of a contract dispute. |
Foreign sales of our Pure-Vu system could also be adversely affected by the imposition of governmental controls, political and economic instability, trade restrictions and changes in tariffs, any of which may adversely affect our results of operations.
| 14 |
We are, and will be, completely dependent on third parties to manufacture our Pure-Vu system, and our commercialization of our Pure-Vu system could be halted, delayed or made less profitable if those third parties fail to obtain manufacturing approval from the FDA or comparable foreign regulatory authorities, fail to provide us with sufficient quantities of our Pure-Vu system device components or fail to do so at acceptable quality levels or prices.
We do not currently have, nor do we plan to acquire, the capability or infrastructure to manufacture our Pure-Vu system, as well as the other related device components, for use in our clinical trials, if required, or for commercial product, if any. In addition, we do not have the capability to produce our Pure-Vu system for commercial distribution. As a result, we will be obligated to rely on contract manufacturers for the commercial supply of our product. We have not entered into an agreement with any contract manufacturers for the commercial supply of our product and we may not be able to engage a contract manufacturer for such supply on favorable terms to us, or at all.
The facilities used by any future contract manufacturers, if any, to manufacture the Pure-Vu system must be approved by the FDA. We do not control the manufacturing process of, and are completely dependent on, any future contract manufacturing partners, if any, for compliance with current Good Manufacturing Practices (“cGMPs”) for manufacture of medical devices. These cGMPs regulations cover all aspects of the manufacturing, testing, quality control and record keeping relating to the Pure-Vu system. If any future contract manufacturers cannot successfully manufacture products that conform to our specifications and the strict regulatory requirements of the FDA or others, they will not be able to secure and/or maintain regulatory approval for their manufacturing facilities. If the FDA or a comparable foreign regulatory authority does not approve these facilities for the manufacture of our products or if it withdraws any such approval in the future, we may need to find alternative manufacturing facilities, which would significantly impact our ability to maintain regulatory approval for or market the Pure-Vu system.
Our future contract manufacturers, if any, will be subject to ongoing periodic unannounced inspections by the FDA and corresponding state and foreign agencies for compliance with cGMPs and similar regulatory requirements. We will not have control over our contract manufacturers’ compliance with these regulations and standards. Failure by any of our contract manufacturers to comply with applicable regulations could result in sanctions being imposed on us, including fines, injunctions, civil penalties, failure to market the Pure-Vu system, delays, suspensions or withdrawals of approvals, operating restrictions and criminal prosecutions, any of which could significantly and adversely affect our business. In addition, we will not have control over the ability of our future contract manufacturers, if any, to maintain adequate quality control, quality assurance and qualified personnel. Failure by our future contract manufacturers, if any, to comply with or maintain any of these standards could adversely affect our ability to maintain regulatory approval for or market our Pure-Vu system.
If, for any reason, these third parties are unable or unwilling to perform, we may not be able to terminate our agreements, if any, with them, and we may not be able to locate alternative manufacturers or enter into favorable agreements with them and we cannot be certain that any such third parties will have the manufacturing capacity to meet future requirements. If these future manufacturers or any alternate manufacturer experiences any significant difficulties in its respective manufacturing processes for our product or should cease doing business with us, we could experience significant interruptions in supply or may not be able to create or maintain a commercial supply. Were we to encounter manufacturing issues, our ability to produce sufficient commercial supply might be negatively affected. Our inability to coordinate the efforts of our future third party manufacturing partners, if any, or the lack of capacity available at our third party manufacturing partners, could impair our ability to supply our Pure-Vu system at required levels. If we face these or other difficulties with our future manufacturing partners, if any, we could experience significant interruptions in the supply of our products if we decided to transfer the manufacture to one or more alternative manufacturers in an effort to deal with the difficulties.
Any manufacturing problem or the loss of a contract manufacturer could be disruptive to our operations and result in lost sales. Any reliance on suppliers may involve several risks, including a potential inability to obtain critical components and reduced control over production costs, delivery schedules, reliability and quality. Any unanticipated disruption to a future contract manufacturer caused by problems at suppliers could delay shipment of our Pure-Vu system, increase our cost of goods sold and result in lost sales.
| 15 |
The manufacture of our Pure-Vu system, and the technology developed thereunder, is subject to certain Israeli government regulations which may impair our ability to outsource or transfer development or manufacturing activities with respect to any product or technology outside of Israel.
We have received, and may receive in the future, grants from the Government of the State of Israel through the Israel Innovation Authority of the Ministry of Economy and Industry (the “IIA”) (formerly known as the Office of the Chief Scientist of the Ministry of Economy and Industry (the “OCS”)), for the financing of a portion of our research and development expenditures pursuant to the Encouragement of Research, Development and Technological Innovation in the Industry Law 5744-1984 (formerly known as the Encouragement of Industrial Research and Development Law, 5744-1984), referred to as the Research Law, and related regulations. In exchange for these grants, we are required to pay royalties to the IIA from our revenues on sales of products and services based on technology developed using IIA grants, up to an aggregate of 100% of the U.S. dollar-linked value of the grant (plus interest), which amount may be increased under certain circumstances. The terms of the Israeli government participation also require that products developed with IIA grants be manufactured in Israel and that the technology developed thereunder may not be transferred outside of Israel (even following the full repayment of the IIA grants), unless prior approval is received from the IIA, which approval may not be granted. Even if such approval is granted, the transfer outside of Israel of manufacturing which is connected with the IIA-funded know-how may result in increased royalties (up to three times the aggregate amount of the IIA grants plus interest thereon), as well as in a higher royalty repayment rate. In addition, the transfer outside of Israel of IIA-funded know-how may trigger additional payments to the IIA (up to six times the aggregate amount of the IIA grants plus interest thereon). Even following the full repayment of any IIA grants, we must nevertheless continue to comply with the requirements of the Research Law. The foregoing restrictions may impair our ability to outsource or transfer development or manufacturing activities with respect to any product or technology outside of Israel. For additional information, see “Risks Related to Our Operations in Israel.”
A significant amendment to the Research Law entered into effect on January 1, 2016, under which the IIA, a statutory government corporation, was established, which replaced the OCS. Under such amendment, the IIA is authorized to establish rules concerning the ownership and exploitation of IIA/OCS-funded know-how (including with respect to restrictions on transfer of manufacturing activities and IIA-funded know-how outside of Israel), which may differ from the restrictive laws, regulations and guidelines as currently in effect (and which shall remain in effect until such rules have been established by the IIA). In May 2017, the IIA issued new rules applicable to Israeli companies that receive grants from the IIA or its predecessor, the OCS, which went into effect on July 1, 2017. As of the date hereof, we cannot predict the impact these new rules will have on us or how they will be implemented by the IIA. Furthermore, it is anticipated that additional rules will be published by the IIA and we cannot predict or estimate the changes (if any) that may be made to this legislation (including with respect to the acquisition of an IIA-funded entity or the transfer of manufacturing or ownership of IIA-funded technology).
Risks Relating to Our Intellectual Property Rights
We may be unable to protect our intellectual property rights or may infringe on the intellectual property rights of others.
We rely on a combination of patents, trademarks, copyrights, trade secrets and nondisclosure agreements to protect our proprietary intellectual property. Our efforts to protect our intellectual property and proprietary rights may not be sufficient. We cannot be sure that our pending patent applications will result in the issuance of patents to us, that patents issued to or licensed by us in the past or in the future will not be challenged or circumvented by competitors or that these patents will remain valid or sufficiently broad to preclude our competitors from introducing technologies similar to those covered by our patents and patent applications. In addition, our ability to enforce and protect our intellectual property rights may be limited in certain countries outside the United States, which could make it easier for competitors to capture market position in such countries by utilizing technologies that are similar to those developed or licensed by us.
Competitors also may harm our sales by designing products that mirror the capabilities of our products or technology without infringing our intellectual property rights. If we do not obtain sufficient protection for our intellectual property, or if we are unable to effectively enforce our intellectual property rights, our competitiveness could be impaired, which would limit our growth and future revenue.
We operate in an industry characterized by extensive patent litigation. Patent litigation is costly to defend and can result in significant damage awards, including treble damages under certain circumstances, and injunctions that could prevent the manufacture and sale of affected products or force us to make significant royalty payments in order to continue selling the affected products.
| 16 |
We may be subject to claims that we have wrongfully hired an employee from a competitor or that we or our employees have wrongfully used or disclosed alleged confidential information or trade secrets of their former employers.
As is commonplace in our industry, we employ and plan to employ individuals who were previously employed at other medical device companies, including our competitors or potential competitors. Although no claims against us are currently pending, we may be subject in the future to claims that our employees or prospective employees are subject to a continuing obligation to their former employers (such as non-competition or non-solicitation obligations) or claims that our employees or we have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of their former employers. Litigation may be necessary to defend against these claims. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to management.
General Company-Related Risks
We will need to grow the size of our organization, and we may experience difficulties in managing this growth.
As of September 30, 2017, we had 36 full time employees. As our marketing and commercialization plans and strategies develop, we will need to expand the size of our employee and consultant base for managerial, operational, sales, marketing, financial and other resources. Future growth would impose significant added responsibilities on members of management, including the need to identify, recruit, maintain, motivate and integrate additional employees. In addition, our management may have to divert a disproportionate amount of its attention away from our day-to-day activities and devote a substantial amount of time to managing these growth activities. Our future financial performance and our ability to commercialize the Pure-Vu system and any other future product candidates and our ability to compete effectively will depend, in part, on our ability to effectively manage our future growth.
Our success will depend in part on our ability to manage our operations as we advance our product candidates through clinical trials and to expand our development or regulatory capabilities or contract with third parties to provide these capabilities for us. Failure to achieve any of these goals could have a material adverse effect on our business, financial condition or results of operations.
Future capital raises may dilute our existing stockholders’ ownership and/or have other adverse effects on our operations.
If we raise additional capital by issuing equity securities, our existing stockholders’ percentage ownership will be reduced and these stockholders may experience substantial dilution. If we raise additional funds by issuing debt securities, these debt securities would have rights senior to those of our common stock and the terms of the debt securities issued could impose significant restrictions on our operations, including liens on our assets. If we raise additional funds through collaborations and licensing arrangements, we may be required to relinquish some rights to our technologies or products, or to grant licenses on terms that are not favorable to us.
If we are not successful in attracting and retaining highly qualified personnel, we may not be able to successfully implement our business strategy. In addition, the loss of the services of certain key employees would adversely impact our business prospects.
We depend on key members of our management team. The loss of the services of Mark Pomeranz, our Chief Executive Officer, Andrew Taylor, our Chief Financial Officer or any member of our senior management team, could harm our ability to execute our commercial strategy for our Pure-Vu system and the strategic objectives for our company. In connection with the Share Exchange Transaction, we entered into an employment agreement with our Chief Executive Officer, but this agreement is terminable by Mr. Pomeranz on short or no notice at any time without penalty. We also entered into an employment agreement with our Chief Financial Officer, and this agreement is also terminable by Mr. Taylor on short or no notice at any time without penalty. In addition, we do not maintain, and have no current intention of obtaining, “key man” life insurance on any member of our management team.
| 17 |
Recruiting and retaining qualified scientific and commercial personnel, including sales and marketing executives and field personnel, is also critical to our success. We may not be able to attract and retain these personnel on acceptable terms given the competition among numerous medical device and pharmaceutical companies for similar personnel and based on our company profile. We also experience competition for the hiring of scientific personnel from universities and research institutions. If we fail to recruit and then retain these personnel, we may not be able to effectively execute our commercial strategy for the Pure-Vu system.
If product liability lawsuits are brought against us, we may incur substantial liabilities and may be required to limit commercialization of the Pure-Vu system.
We are, and may be in the future, subject to product liability claims and lawsuits, including potential class actions, alleging that our products have resulted or could result in an unsafe condition or injury. Any product liability claim brought against us, with or without merit, could be costly to defend and could result in settlement payments and adjustments not covered by or in excess of insurance. In addition, we may not be able to obtain insurance on terms acceptable to us or at all because insurance varies in cost and can be difficult to obtain. Our failure to successfully defend against product liability claims or maintain adequate insurance coverage could have an adverse effect on our results of operations and financial condition.
Exchange rate fluctuations between the U.S. dollar and the Israeli New Shekel (the “NIS”) and inflation may negatively affect our earnings and we may not be able to hedge our currency exchange risks successfully.
The U.S. dollar is our functional and reporting currency. However, a significant portion of our operating expenses, including personnel and facilities related expenses, are incurred in NIS. As a result, we are exposed to the risks that the NIS may appreciate relative to the U.S. dollar, or, if the NIS instead devalues relative to the U.S. dollar, that the inflation rate in Israel may exceed such rate of devaluation of the NIS, or that the timing of such devaluation may lag behind inflation in Israel. In any such event, the dollar cost of our operations in Israel would increase and our dollar-denominated results of operations would be adversely affected. Given our general lack of currency hedging arrangements to protect us from fluctuations in the exchange rates of the NIS and other foreign currencies in relation to the U.S. dollar (and/or from inflation of such foreign currencies), we may be exposed to material adverse effects from such movements. We cannot predict any future trends in the rate of inflation in Israel or the rate of devaluation (if any) of the NIS against the U.S. dollar.
We may acquire other businesses or form joint ventures or make investments in other companies or technologies that could negatively affect our operating results, dilute our stockholders’ ownership, increase our debt or cause us to incur significant expense.
We may pursue acquisitions of businesses and assets. We also may pursue strategic alliances and joint ventures that leverage our intellectual property and industry experience to expand our offerings or distribution. We have no history of acquiring other companies or with forming strategic partnerships. We may not be able to find suitable partners or acquisition candidates, and we may not be able to complete such transactions on favorable terms, if at all. If we make any acquisitions, we may not be able to integrate these acquisitions successfully into our existing business, and we could assume unknown or contingent liabilities. Any future acquisitions also could result in the issuance of equity securities, incurrence of debt, contingent liabilities or future write-offs of intangible assets or goodwill, any of which could have a material adverse effect on our financial condition, results of operations, and cash flows. Integration of an acquired company also may disrupt ongoing operations and require management resources that we would otherwise focus on developing our existing business. We may experience losses related to investments in other companies, which could have a material negative effect on our results of operations and financial condition. We may not realize the anticipated benefits of any acquisition, technology license, strategic alliance, or joint venture.
| 18 |
To finance any acquisitions or joint ventures, we may choose to issue shares of our common stock as consideration, which would dilute the ownership of our stockholders. Additional funds may not be available on terms that are favorable to us, or at all. If the price of our common stock is low or volatile, we may not be able to acquire other companies or fund a joint venture project using our stock as consideration.
Risks Related to our Capital Stock
We have engaged in transactions with the Placement Agent in our 2017 Private Placement and its related parties that could present conflicts of interest.
There have been transactions between us and related parties of the Placement Agent that could present potential conflicts of interest. These transactions include, but are not limited to, (i) the engagement of the Placement Agent to assist in Opco’s sale of Convertible Notes and Convertible Note Warrants in October and November 2016 and the payment of compensation in the form of cash, warrants, and a non-accountable expense allowance, (ii) the issuance of founders shares of the Company to affiliates of the Placement Agent and (iii) the engagement of the Placement Agent as Placement Agent in the 2017 Private Placement and the payment of compensation in the form of cash, warrants, royalty payment obligations and a non-accountable expense allowance. Each of these and other present and future financial commitments or agreements could constitute potential conflicts of interest.
After this offering, our officers, directors, and principal stockholders will continue to exercise significant control over our Company, and may control our company for the foreseeable future, including the outcome of matters requiring stockholder approval.
When this offering is completed, our officers, directors, entities controlled by our officers and directors, and principal stockholders who beneficially own more than 5% of our common stock before this offering will in the aggregate, beneficially own shares representing approximately % of our outstanding capital stock immediately after this offering. As a result, such entities and individuals may have the ability, acting together, to control the election of our directors and the outcome of corporate actions requiring stockholder approval, such as: (i) a merger or a sale of our company, (ii) a sale of all or substantially all of our assets, and (iii) amendments to our certificate of incorporation and bylaws. This concentration of voting power and control could have a significant effect in delaying, deferring or preventing an action that might otherwise be beneficial to our other stockholders and be disadvantageous to our stockholders with interests different from those entities and individuals. These individuals also have significant control over our business, policies and affairs as officers and directors of our company. Therefore, you should not invest in reliance on your ability to have any control over our company.
Our quarterly operating results may be subject to significant fluctuations.
To date, as part of our limited launch, we have generated some revenue from our Pure-Vu system, but we do not expect to generate significant revenue from product sales unless and until we expand our commercialization efforts, which we expect will take a number of years and is subject to significant uncertainty, and accordingly we may experience significant fluctuations in our quarterly operating results in the future. The rate of market acceptance of our Pure-Vu system could contribute to this quarterly variability. Our limited operating history complicates our ability to project quarterly revenue and any future revenue generated from sales of our Pure-Vu system may fluctuate from time to time. In addition, our expense levels are based, in part, on expectation of future revenue levels. A shortfall in expected revenue, if any, could, therefore, result in a disproportionate decrease in our net income. As a result, our quarterly operating results may be subject to significant fluctuations.
Future sales of shares by existing stockholders could cause our stock price to decline.
If our existing stockholders sell, or indicate an intent to sell, substantial amounts of our common stock in the public market after the 180-day contractual lock-up and other legal restrictions on resale discussed in this prospectus lapse, the trading price of our common stock could decline significantly and could decline below the initial public offering price. Based on 10,491,841 shares outstanding as of September 30, 2017, upon the completion of this offering, we will have outstanding shares of common stock, assuming no exercise of outstanding options, warrants or conversion of Series A Convertible Preferred Stock. Of these shares, shares of common stock, plus any shares sold pursuant to the underwriters’ option to purchase additional shares, will be immediately freely tradable, without restriction, in the public market.
| 19 |
After the lock-up agreements pertaining to this offering expire and based on shares outstanding after this offering, an additional shares will be eligible for sale in the public market. In addition, upon issuance, the 1,340,869 shares subject to the exercise of outstanding warrants, 1,581,128 shares subject to conversion of outstanding Series A Convertible Preferred Stock, 1,836,844 shares subject to outstanding options under our stock option plans, and the 169,812 shares reserved for future issuance under our equity compensation plan, all as of September 30, 2017, will become eligible for sale in the public market in the future, subject to certain legal and contractual limitations. If our existing stockholders sell substantial amounts of our common stock in the public market, or if the public perceives that such sales could occur, this could have an adverse impact on the market price of our common stock, even if there is no relationship between such sales and the performance of our business.
We will have broad discretion in how we use the net proceeds of this offering. We may not use these proceeds effectively, which could affect our results of operations and cause our stock price to decline.
We will have considerable discretion in the application of the net proceeds of this offering, including for any of the purposes described in the section entitled “Use of Proceeds.” We intend to use the net proceeds from this offering for commercialization of our Pure-Vu system, research and development activities, working capital and other general corporate purposes. As a result, investors will be relying upon management’s judgment with only limited information about our specific intentions for the use of the balance of the net proceeds of this offering. We may use the net proceeds for purposes that do not yield a significant return or any return at all for our stockholders. In addition, pending their use, we may invest the net proceeds from this offering in a manner that does not produce income or that loses value.
If equity research analysts do not publish research or reports about our business or if they issue unfavorable commentary or downgrade our common stock, the price of our common stock could decline.
The trading market for our common stock will rely in part on the research and reports that equity research analysts publish about us and our business. We do not control these analysts. The price of our common stock could decline if one or more equity analysts downgrade our common stock or if analysts issue other unfavorable commentary or cease publishing reports about us or our business.
Investors in this offering will pay a higher price than the book value of our common stock.
If you purchase common stock in this offering, you will pay more for your shares than the amounts paid by existing stockholders for their shares. You will incur immediate and substantial dilution of $ per share, representing the difference between our pro forma net tangible book value per share after giving effect to this offering and an assumed initial public offering price of $ per share, the midpoint of the estimated price range set forth on the cover of this prospectus. In the past, we issued Series A Convertible Preferred Stock, restricted stock, options and warrants to acquire or convert into capital stock at prices significantly below the assumed initial public offering price. To the extent any outstanding Series A Convertible Preferred Stock, options or warrants are ultimately converted or exercised, you will sustain further dilution. For further information on this calculation, see the section entitled “Dilution.”
| 20 |
No public market for our common stock currently exists, and an active trading market may not develop or be sustained following this offering.
Prior to this offering, there has been no public market for our common stock. An active trading market may not develop following the completion of this offering or, if developed, may not be sustained. Certain of our existing stockholders, including stockholders affiliated with certain of our directors, have indicated an interest in purchasing up to an aggregate of approximately $ million in shares of our common stock in this offering at the initial public offering price. To the extent these potential investors are allocated and purchase shares in this offering, such purchases would reduce the available public float for our shares because these potential investors will be restricted from selling the shares under the lock-up agreements described in the “Shares Eligible for Future Sale” section of this prospectus. As a result, the liquidity of our common stock could be significantly reduced from what it would have been if these shares had been purchased by investors that were not affiliated with us. The lack of an active market may impair your ability to sell your shares at the time you wish to sell them or at a price that you consider reasonable. The lack of an active market may also reduce the fair market value of your shares. An inactive market may also impair our ability to raise capital to continue to fund operations by selling shares and may impair our ability to acquire additional intellectual property assets by using our shares as consideration.
Our share price may be volatile, which could subject us to securities class action litigation and prevent you from being able to sell your shares at or above the offering price.
The initial public offering price for our shares will be determined by negotiations between us and the underwriters based on several factors. This price may vary from the market price of our common stock after this offering. You may be unable to sell your shares of common stock at or above the initial offering price. The market price for our common stock may be volatile and subject to wide fluctuations in response to factors including the following:
| ● | actual or anticipated fluctuations in our quarterly or annual operating results; | |
| ● | actual or anticipated changes in our growth rate relative to our competitors; | |
| ● | failure to meet or exceed financial estimates and projections of the investment community or that we provide to the public; | |
| ● | issuance of new or updated research or reports by securities analysts; | |
| ● | share price and volume fluctuations attributable to inconsistent trading volume levels of our shares; additions or departures of key management or other personnel; | |
| ● | disputes or other developments related to proprietary rights, including patents, litigation matters, and our ability to obtain patent protection for our technologies; | |
| ● | announcement or expectation of additional debt or equity financing efforts; | |
| ● | sales of our common stock by us, our insiders or our other stockholders; and | |
| ● | general economic, market or political conditions in the United States or elsewhere. |
In particular, the market prices of early commercial-stage companies like ours have been highly volatile due to factors, including, but not limited to:
| ● | any delay or failure to conduct a clinical trial for our product or receive approval from the FDA and other regulatory agents; | |
| ● | developments or disputes concerning our product’s intellectual property rights; | |
| ● | our or our competitors’ technological innovations; | |
| ● | fluctuations in the valuation of companies perceived by investors to be comparable to us; | |
| ● | announcements by us or our competitors of significant contracts, acquisitions, strategic partnerships, joint ventures, capital commitments, new technologies or patents; | |
| ● | failure to complete significant transactions or collaborate with vendors in manufacturing our product; and | |
| ● | proposals for legislation that would place restrictions on the price of medical therapies. |
These and other market and industry factors may cause the market price and demand for our common stock to fluctuate substantially, regardless of our actual operating performance, which may limit or prevent investors from readily selling their shares of common stock and may otherwise negatively affect the liquidity of our common stock. In addition, the stock market in general, and NASDAQ Capital Markets and emerging growth companies in particular, have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of these companies. In the past, when the market price of a stock has been volatile, holders of that stock have instituted securities class action litigation against the company that issued the stock. If any of our stockholders brought a lawsuit against us, we could incur substantial costs defending the lawsuit. Such a lawsuit could also divert the time and attention of our management.
| 21 |
Shareholders will experience dilution by conversion of preferred stock and exercises of outstanding warrants and options.
As of September 30, 2017, there are 1,581,128 shares of our common stock issuable upon the conversion of outstanding Series A Convertible Preferred Stock. As of September 30, 2017, there are 1,310,869 shares of our common stock issuable upon the exercise of Exchange Warrants (defined below, see “Business - Exchange of Convertible Note Warrants”) and Placement Agent Warrants (defined below, see “Business - 2017 Private Placement and Convertible Note Offering – Placement Agent Compensation”), each at an exercise price of $5.00 per share, 30,000 shares of our common stock issuable upon the exercise of warrants issued to a service provider, at an exercise price of $8.00 per share, and options to purchase an aggregate of up to 1,836,844 shares of our common stock, at exercise prices ranging from $2.38 to $4.50, following the modification of share-based payment awards on September 29, 2017.
The conversion of such preferred stock and the exercise of such warrants and options will result in dilution of your investment. As a result of this dilution, you may receive significantly less than the full purchase price you paid for securities of the Company in the event of liquidation.
Pursuant to the terms of our outstanding Series A Preferred Stock, we may be obligated to pay significant royalties.
Pursuant to the terms of the Certificate of Designations of Preferences, Rights and Limitations of Series A Convertible Preferred Stock (the “Certificate of Designations”) for our outstanding Series A Convertible Preferred Stock, and pursuant to the terms of the Placement Agent royalty payment rights certificates (the “Placement Agent Royalty Payment Rights Certificates”) issued in connection with the 2017 Private Placement, we may be required to make certain royalty payments. If and when we generate sales of the current and potential future versions of the Pure-Vu system, including disposables, parts, and services, or if we receive any proceeds from the licensing of the current and potential future versions of the Pure-Vu system, then we are required to pay to the holders of our Series A Convertible Preferred Stock and the holders of the Placement Agent Royalty Payment Rights Certificates, subject to certain vesting requirements, a royalty equal to (i) three percent (3%) of our net sales, if any, in any calendar year, not in excess of $30 million per year and (ii) 5% of our licensing proceeds, if any, in any calendar year, not in excess of $30 million per year. The royalties will be payable up to the later of (i) the latest expiration date for our current patents (which is currently October 2026), or (ii) the latest expiration date of any pending patents as of the date of the Initial Closing that may be issued in the future.
We are an “emerging growth company,” and will be able take advantage of reduced disclosure requirements applicable to “emerging growth companies,” which could make our common stock less attractive to investors.
We are an “emerging growth company,” as defined in the JOBS Act and, for as long as we continue to be an “emerging growth company,” we intend to take advantage of certain exemptions from various reporting requirements applicable to other public companies but not to “emerging growth companies,” including, but not limited to, not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. We could be an “emerging growth company” for up to five years, or until the earliest of (i) the last day of the first fiscal year in which our annual gross revenues exceed $1.07 billion, (ii) the date that we become a “large accelerated filer” as defined in Rule 12b-2 under the Exchange Act, which would occur if the market value of our common stock that is held by non-affiliates exceeds $700 million as of the last business day of our most recently completed second fiscal quarter, or (iii) the date on which we have issued more than $1 billion in non-convertible debt during the preceding three year period.
| 22 |
We intend to take advantage of these reporting exemptions described above until we are no longer an “emerging growth company.” Under the JOBS Act, “emerging growth companies” can also delay adopting new or revised accounting standards until such time as those standards apply to private companies. We have irrevocably elected not to avail ourselves of this exemption from new or revised accounting standards and, therefore, we will be subject to the same new or revised accounting standards as other public companies that are not “emerging growth companies.”
We cannot predict if investors will find our common stock less attractive if we choose to rely on these exemptions. If some investors find our common stock less attractive as a result of any choices to reduce future disclosure, there may be a less active trading market for our common stock and our stock price may be more volatile.
We will incur significantly increased costs and devote substantial management time as a result of operating as a public company particularly after we are no longer an “emerging growth company.”
As a public company, we will incur significant legal, accounting and other expenses that we did not incur as a private company. For example, we will be required to comply with certain of the requirements of the Sarbanes-Oxley Act and the Dodd-Frank Wall Street Reform and Consumer Protection Act, as amended, as well as rules and regulations subsequently implemented by the SEC, including the establishment and maintenance of effective disclosure and financial controls and changes in corporate governance practices. We expect that compliance with these requirements will increase our legal and financial compliance costs and will make some activities more time consuming and costly. In addition, we expect that our management and other personnel will need to divert attention from operational and other business matters to devote substantial time to these public company requirements. In particular, we expect to incur significant expenses and devote substantial management effort toward ensuring compliance with the requirements of Section 404 of the Sarbanes-Oxley Act. In addition, after we no longer qualify as an “emerging growth company,” as defined under the JOBS ACT we expect to incur additional management time and cost to comply with the more stringent reporting requirements applicable to companies that are deemed accelerated filers or large accelerated filers, including complying with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act. We are just beginning the process of compiling the system and processing documentation needed to comply with such requirements. We may not be able to complete our evaluation, testing and any required remediation in a timely fashion. In that regard, we currently do not have an internal audit function, and we will need to hire or contract for additional accounting and financial staff with appropriate public company experience and technical accounting knowledge.
We cannot predict or estimate the amount of additional costs we may incur as a result of becoming a public company or the timing of such costs.
There may be limitations on the effectiveness of our internal controls, and a failure of our control systems to prevent error or fraud may materially harm our company.
Proper systems of internal controls over financial accounting and disclosure controls and procedures are critical to the operation of a public company. As we are a start-up company, we only have 4 employees, and 2 contractors in our finance and accounting functions, which may result in a lack of segregation of duties and are at the very early stages of establishing, and we may be unable to effectively establish such systems, especially in light of the fact that we expect to operate as a publicly reporting company. This would leave us without the ability to reliably assimilate and compile financial information about our company and significantly impair our ability to prevent error and detect fraud, all of which would have a negative impact on our company from many perspectives.
Moreover, we do not expect that disclosure controls or internal control over financial reporting, even if established, will prevent all error and all fraud. A control system, no matter how well designed and operated, can provide only reasonable, not absolute, assurance that the control system’s objectives will be met. Further, the design of a control system must reflect the fact that there are resource constraints and the benefits of controls must be considered relative to their costs. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, have been detected. Failure of our control systems to prevent error or fraud could materially adversely impact us.
| 23 |
We may have a material weakness in our internal control over financial reporting. In addition, because of our status as an emerging growth company, our independent registered public accountants are not required to provide an attestation report as to our internal control over financial reporting for the foreseeable future.
We may be required, pursuant to Section 404 of the Sarbanes-Oxley Act, to furnish a report by our management on, among other things, the effectiveness of our internal control over financial reporting for the first fiscal year beginning after the effective date of the registration statement of which this prospectus is a part. This assessment will need to include disclosure of any material weaknesses identified by our management in our internal control over financial reporting, as well as a statement that our independent registered public accounting firm has issued an opinion on our internal control over financial reporting. We are in the very early stages of the costly and challenging process of compiling the system and processing documentation necessary to perform the evaluation needed to comply with Section 404.
We may not be able to complete our evaluation, testing and any required remediation in a timely fashion. During the evaluation and testing process, if we identify one or more material weaknesses in our internal control over financial reporting, we will be unable to assert that our internal controls are effective.
If we are unable to assert that our internal control over financial reporting is effective, or, if applicable, our independent registered public accounting firm is unable to express an opinion on the effectiveness of our internal controls, we could lose investor confidence in the accuracy and completeness of our financial reports, which would cause the price of our common stock to decline, and we may be subject to investigation or sanctions by the SEC. We will also be required to disclose changes made in our internal control and procedures on a quarterly basis.
However, our independent registered public accounting firm will not be required to formally attest to the effectiveness of our internal control over financial reporting pursuant to Section 404 until the later of the year following our first annual report required to be filed with the SEC, or the date we are no longer an “emerging growth company” as defined in the recently enacted JOBS Act, if we take advantage (as we expect to do) of the exemptions contained in the JOBS Act. We will remain an “emerging growth company” for up to five years, although if the market value of our common stock that is held by non-affiliates exceeds $700 million as of any June 30th before that time, we would cease to be an “emerging growth company” as of the following December 31st. At such time, our independent registered public accounting firm may issue a report that is adverse in the event it is not satisfied with the level at which our controls are documented, designed or operating. Our remediation efforts may not enable us to avoid a material weakness in our internal control over financial reporting in the future.
Any of the foregoing occurrences, should they come to pass, could negatively impact the public perception of our company, which could have a negative impact on our stock price.
We do not currently intend to pay dividends on our common stock in the foreseeable future, and consequently, your ability to achieve a return on your investment will depend on appreciation in the price of our common stock.
We have never declared or paid cash dividends on our common stock and do not anticipate paying any cash dividends to holders of our common stock in the foreseeable future. Consequently, investors must rely on sales of their common stock after price appreciation, which may never occur, as the only way to realize any future gains on their investments. There is no guarantee that shares of our common stock will appreciate in value or even maintain the price at which our stockholders have purchased their shares.
Upon dissolution of our company, you may not recoup all or any portion of your investment.
In the event of a liquidation, dissolution or winding-up of our company, whether voluntary or involuntary, the proceeds and/or assets of our company remaining after giving effect to such transaction, and the payment of all of our debts and liabilities and distributions required to be made to holders of any outstanding preferred stock will then be distributed to the stockholders of our common stock on a pro rata basis. There can be no assurance that we will have available assets to pay to the holders of our common stock, or any amounts, upon such a liquidation, dissolution or winding-up of our Company. In this event, you could lose some or all of your investment.
| 24 |
Our ability to use our net operating loss carryforwards and certain other tax attributes may be limited.
As a result of the Share Exchange Transaction, our ability to utilize our federal net operating loss, carryforwards and federal tax credit may be limited under Sections 382 of the Internal Revenue Code of 1986, as amended (the “Code”). The limitations apply if an “ownership change,” as defined by Section 382, occurs. Generally, an ownership change occurs if the percentage of the value of the stock that is owned by one or more direct or indirect “five percent shareholders” increases by more than 50 percentage points over their lowest ownership percentage at any time during the applicable testing period (typically three years). In addition, future changes in our stock ownership, which may be outside of our control, may trigger an “ownership change” and, consequently, Section 382 limitations. As a result, if we earn net taxable income, our ability to use our pre-change net operating loss carryforwards and other tax attributes to offset United States federal taxable income may be subject to limitations, which could potentially result in increased future tax liability to us.
Our certificate of incorporation allows for our board to create new series of preferred stock without further approval by our stockholders, which could adversely affect the rights of the holders of our common stock.
Our board of directors has the authority to fix and determine the relative rights and preferences of preferred stock. We anticipate that our board of directors will have the authority to issue up to 10 million shares of our preferred stock without further stockholder approval. As a result, our board of directors could authorize the issuance of a series of preferred stock that would grant to holders the preferred right to our assets upon liquidation and the right to receive dividend payments before dividends are distributed to the holders of our common stock. In addition, our board of directors could authorize the issuance of a series of preferred stock that has greater voting power than our common stock or that is convertible into our common stock, which could decrease the relative voting power of our common stock or result in dilution to our existing stockholders.
Our certificate of incorporation, as amended, designates the Court of Chancery of the State of Delaware as the sole and exclusive forum for certain types of actions and proceedings that may be initiated by our stockholders, which could discourage lawsuits against us, and our directors and officers.
Our certificate of incorporation, as amended, provides that unless we consent in writing to the selection of an alternative forum, the Court of Chancery of the State of Delaware will be the sole and exclusive forum for (i) any derivative action or proceeding brought on our behalf; (ii) any action asserting a claim of breach of a fiduciary duty; (iii) any action asserting a claim against us, or any of our officers or directors, arising pursuant to, or a claim against us, or any of our officers or directors, with respect to the interpretation or application of any provision of the Delaware General Corporation Law, our certificate of incorporation, as amended, or our bylaws; or (iv) any action asserting a claim governed by the internal affairs doctrine. However, if the Court of Chancery of the State of Delaware dismisses any such action for lack of subject matter jurisdiction, the action may be brought in another state court sitting in the State of Delaware.
Risks Related to Our Operations in Israel
Our principal offices, research and development facilities and some of our suppliers are located in Israel and, therefore, our business, financial condition and results of operation may be adversely affected by political, economic and military instability in Israel.
Our principal offices, research and development facilities are located in northern Israel. In addition, most of our employees are residents of Israel. Accordingly, political, economic and military conditions in Israel may directly affect our business. Since the State of Israel was established in 1948, the State of Israel and its economy has experienced significant growth and expansion, coupled with an increase in the standard of living, and has developed one of the most advanced high-tech industries in the world. However, it continues to face many geo-political and other challenges that may affect companies located in Israel, such as ours. For example, a number of armed conflicts have occurred between Israel and its Arab neighbors. Although Israel has entered into various peace agreements with Egypt and Jordan as well as comprehensive agreements with the Palestinian Authority, there continues to be unrest and terrorist activity in Israel with varying levels of severity, as well as ongoing hostilities and armed conflicts between Israel and the Palestinian Authority and other groups in the West Bank and Gaza Strip. The effects of these hostilities and violence on the Israeli economy and our operations are unclear, and we cannot predict the effect on us of a further increase in these hostilities or any future armed conflict, political instability or violence in the region. We could be harmed by any major hostilities involving Israel, the interruption or curtailment of trade between Israel and its trading partners, boycotts or a significant downturn in the economic or financial condition of Israel. The impact of Israel’s relations with its Arab neighbors in general, or on our operations in the region in particular, remains uncertain. The establishment of new fundamentalist Islamic regimes or governments more hostile to Israel could have serious consequences for the stability in the region, place additional political, economic and military confines upon Israel, materially adversely affect our operations and limit our ability to sell our products to countries in the region.
| 25 |
Additionally, several countries, principally in the Middle East, still restrict doing business with Israel and Israeli companies, and additional countries and groups have imposed or may impose restrictions on doing business with Israel and Israeli companies if hostilities in Israel or political instability in the region continues or increases. These restrictions may limit our ability to sell our products to companies in these countries. Furthermore, the Boycott, Divestment and Sanctions Movement, a global campaign attempting to increase economic and political pressure on Israel to comply with the stated goals of the movement, may gain increased traction and result in a boycott of Israeli products and services. Any hostilities involving Israel or the interruption or curtailment of trade between Israel and its present trading partners, or significant downturn in the economic or financial condition of Israel, could adversely affect our business, results of operations and financial condition.
Our commercial insurance policy does not cover losses associated with armed conflicts and terrorist attacks. Although the Israeli government in the past covered the reinstatement value of certain damages that were caused by terrorist attacks or acts of war, we cannot assure you that this government coverage will be maintained, or if maintained, will be sufficient to compensate us fully for damages incurred. Any losses or damages incurred by us could have a material adverse effect on our business.
Our operations could also be disrupted by the obligations of personnel to perform military service. Some of our employees in Israel may be called upon to perform up to 54 days in each three year period (and in the case of military officers, up to 84 days in each three year period) of military reserve duty until they reach the age of 40 (and in some cases, depending on their specific military profession up to 45 or even 49 years of age) and, in certain emergency circumstances, may be called to immediate and unlimited active duty. In response to increases in terrorist activity, there have been periods of significant call-ups of military reservists and it is possible that there will be similar large-scale military reserve duty call-ups in the future. Our operations could be disrupted by the absence of a significant number of employees related to military service, which could materially adversely affect our business and results of operations.
Pursuant to the terms of the Israeli government grants we received for research and development expenditures, we are obligated to pay certain royalties on our revenues to the Israeli government. The terms of the grants require us to satisfy specified conditions and to make additional payments in addition to repayment of the grants upon certain events.
We have received grants from the Government of the State of Israel through the IIA (formerly known as the OCS) for the financing of a portion of our research and development expenditures pursuant to the Research Law and related regulations. As of September 30, 2017, we had received funding from the IIA in the aggregate amount of $1.33 million and had a contingent obligation to the IIA up to an aggregate amount of approximately $1.41 million, which is generally repaid in the form of royalties ranging from 3% to 3.5% of revenues. As of September 30, 2017, we paid a minimal amount to the IIA. We may apply for additional IIA grants in the future. However, as the funds available for IIA grants out of the annual budget of the State of Israel have been reduced in the past and may be further reduced in the future, we cannot predict whether we will be entitled to any future grants, or the amounts of any such grants.
In exchange for these grants, we are required to pay the IIA royalties of 3% to 3.5% from our revenues on sales of products and services based on technology developed using IIA grants, up to an aggregate of 100% (which may be increased under certain circumstances) of the U.S. dollar-linked value of the grant, plus interest at the rate of 12-month LIBOR.
| 26 |
The terms of the Israeli government participation also require that products developed with IIA grants be manufactured in Israel and that the technology developed thereunder may not be transferred outside of Israel (including by way of certain licenses), unless prior approval is received from the IIA, which we may not receive. In addition, payment of additional amounts may be required if manufacturing is moved outside of Israel, in which case the royalty repayment rate is increased and the royalty ceiling can reach up to three times the amount of the grants received, and if IIA developed know-how is transferred outside of Israel, the royalty ceiling can reach up to six times the amount of grants received (plus interest). Even following the full repayment of any IIA grants, we must nevertheless continue to comply with the requirements of the Research Law. The foregoing restrictions and requirements for payment may impair our ability to sell our technology assets outside of Israel or to outsource or transfer development or manufacturing activities with respect to any IIA-funded product or technology outside of Israel.
If we fail to comply with any of the conditions and restrictions imposed by the Research Law, or by the specific terms under which we received the grants, we may be required to refund any grants previously received together with interest and penalties, and, in certain circumstances, may be subject to criminal charges.
A significant amendment to the Research Law entered into effect on January 1, 2016 and changed the structure of the OCS, to operate as a governmental corporation entitled the Israeli Innovation Authority or the IIA. Under such amendment, the IIA is authorized to determine rules concerning the ownership and exploitation of IIA/OCS-funded know-how (including with respect to restrictions on transfer of manufacturing activities and IIA-funded know-how outside of Israel), which may differ from the restrictive rules that apply today (which will remain in effect until any such rules have been established by the IIA). In May 2017, the IIA issued new rules applicable to Israeli companies that receive grants from the IIA or its predecessor, the OCS, which went into effect on July 1, 2017. As of the date hereof, we cannot predict the impact these new rules will have on us or how they will be implemented by the IIA. Furthermore, it is anticipated that additional rules will be published by the IIA and we cannot predict or estimate the changes (if any) that may be made to this legislation (including with respect to the acquisition of an IIA-funded entity or the transfer of manufacturing or ownership of IIA-funded technology).
It may be difficult to enforce a judgment of a U.S. court against us or the Israeli experts named in this prospectus in Israel or the United States, to assert U.S. securities laws claims in Israel or to serve process on these experts.
Opco is incorporated in Israel. Our Israeli experts reside in Israel, and substantially all of our assets are located in Israel. Therefore, a judgment obtained against us, or any of such persons may not be collectible in the United States and may not be enforced by an Israeli court. It also may be difficult for you to effect service of process on such persons in the United States or to assert U.S. securities law claims in original actions instituted in Israel. Israeli courts may refuse to hear a claim based on an alleged violation of U.S. securities laws on the grounds that Israel is not the most appropriate forum in which to bring such a claim. In addition, even if an Israeli court agrees to hear a claim, it may determine that Israeli law and not U.S. law is applicable to the claim. If U.S. law is found to be applicable, the content of applicable U.S. law must be proven as a fact by expert witnesses, which can be a time consuming and costly process. Certain matters of procedure would also be governed by Israeli law. There is little binding case law in Israel that addresses the matters described above. As a result of the difficulty associated with enforcing a judgment against us in Israel, you may not be able to collect any damages awarded by either a U.S. or foreign court.
We may become subject to claims for payment of compensation for assigned service inventions by our current or former employees, which could result in litigation and adversely affect our business.
Under the Israeli Patents Law, 5727-1967, or the Patents Law, inventions conceived by an employee during the scope of his or her employment are regarded as “service inventions” and are owned by the employer, absent a specific agreement between the employee and employer giving the employee service invention rights. The Patents Law also provides that if no such agreement between an employer and an employee exists, which prescribes whether, to what extent, and on what conditions the employee is entitled to remuneration for his or her service inventions, then such matters may, upon application by the employee, be decided by a government-appointed compensation and royalties committee established under the Patents Law. A significant portion of our intellectual property has been developed by our employees in Israel in the course of their employment. Such employees have agreed to waive and assign to us all rights to any intellectual property created in the scope of their employment with us, and most of our current employees, including all those involved in the development of our intellectual property, have agreed to waive economic rights they may have with respect to service inventions.
However, despite such contractual obligations, we cannot assure you that claims will not be brought against us by current or former employees demanding remuneration in consideration for assigned alleged service inventions or any other intellectual property rights. If any such claims were filed, we could potentially be required to pay remuneration to our current or former employees for such assigned service inventions or any other intellectual property rights, or be forced to litigate such claims, which could negatively affect our business.
IN ADDITION TO THE ABOVE RISKS, BUSINESSES ARE OFTEN SUBJECT TO RISKS NOT FORESEEN OR FULLY APPRECIATED BY OUR MANAGEMENT. IN REVIEWING THIS PROSPECTUS, POTENTIAL INVESTORS SHOULD KEEP IN MIND THAT THERE MAY BE OTHER POSSIBLE RISKS THAT COULD BE IMPORTANT.
| 27 |
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains, and our officers and representatives may from time to time make, “forward-looking statements,” which include information relating to future events, future financial performance, financial projections, strategies, expectations, competitive environment and regulation. Words such as “may,” “should,” “could,” “would,” “predicts,” “potential,” “continue,” “expects,” “anticipates,” “future,” “intends,” “plans,” “believes,” “estimates,” “goal,” “seek,” “project,” “strategy,” “likely,” and similar expressions, as well as statements in future tense, identify forward-looking statements. Forward-looking statements are neither historical facts, nor should they be read as a guarantee of future performance or results and may not be accurate indications of when such performance or results will be achieved. Forward-looking statements are based on information we have when those statements are made or management’s good faith belief as of that time with respect to future events, and are subject to risks and uncertainties that could cause actual performance or results to differ materially from those expressed in or suggested by the forward-looking statements. Important factors that could cause such differences include, but are not limited to:
| ● | our limited operating history; | |
| ● | our history of operating losses in each year since inception and expectation that we will continue to incur operating losses for the foreseeable future; | |
| ● | our current and future capital requirements to support our development and commercialization efforts for the Pure-Vu system and our ability to satisfy our capital needs; | |
| ● | our dependence on the Pure-Vu system, our sole product candidate, which is still in development; | |
| ● | our ability to obtain approval from regulatory agents in different jurisdictions for the Pure-Vu system; | |
| ● | our Pure-Vu system and the procedure to cleanse the colon in preparation for colonoscopy are not currently reimbursable through private or governmental third-party payors; | |
| ● | our lack of a developed sales and marketing organization and our ability to commercialize the Pure-Vu system; | |
| ● | our dependence on third-parties to manufacture the Pure-Vu system; | |
| ● | our ability to maintain or protect the validity of our patents and other intellectual property; | |
| ● | our ability to retain key executives and medical and science personnel; | |
| ● | our ability to internally develop new inventions and intellectual property; | |
| ● | interpretations of current laws and the passages of future laws; | |
| ● | acceptance of our business model by investors; | |
| ● | the accuracy of our estimates regarding expenses and capital requirements; and | |
| ● | our ability to adequately support growth. |
The foregoing does not represent an exhaustive list of matters that may be covered by the forward-looking statements contained herein or risk factors that we are faced with that may cause our actual results to differ from those anticipate in our forward-looking statements. Please see “Risk Factors” beginning on page 6 for additional risks which could adversely impact our business and financial performance.
Moreover, new risks regularly emerge and it is not possible for our management to predict or articulate all risks we face, nor can we assess the impact of all risks on our business or the extent to which any risk, or combination of risks, may cause actual results to differ from those contained in any forward-looking statements. The Private Securities Litigation Reform Act of 1995 and Section 27A of the Securities Act of 1933, as amended, do not protect any forward-looking statements that we make in connection with this offering. All forward-looking statements included in this prospectus are based on information available to us on the date of this prospectus. Except to the extent required by applicable laws or rules, we undertake no obligation to publicly update or revise any forward-looking statement, whether written or oral, that may be made from time to time, whether as a result of new information, future events or otherwise. All subsequent written and oral forward-looking statements attributable to us or persons acting on our behalf are expressly qualified in their entirety by the cautionary statements contained above and throughout this prospectus. We qualify all of our forward-looking statements by these cautionary statements.
IN ADDITION TO THE ABOVE RISKS, BUSINESSES ARE OFTEN SUBJECT TO RISKS NOT FORESEEN OR FULLY APPRECIATED BY OUR MANAGEMENT. IN REVIEWING THIS PROSPECTUS, POTENTIAL INVESTORS SHOULD KEEP IN MIND THAT THERE MAY BE OTHER POSSIBLE RISKS THAT COULD BE IMPORTANT.
| 28 |
We estimate that we will receive net proceeds of approximately $ million from the sale of the shares of common stock offered in this offering, or approximately $ million if the underwriters exercise their over-allotment option in full, based on an assumed initial public offering price of $ per share (the midpoint of the estimated price range set forth on the cover page of this prospectus) and after deducting the underwriting discounts and commissions and estimated offering expenses payable by us.
Each $1.00 increase (decrease) in the assumed initial public offering price of $ per share (the midpoint of the estimated price range set forth on the cover page of this prospectus) would increase (decrease) the net proceeds to us from this offering, after deducting the underwriting discounts and commissions and estimated offering expenses payable by us, by approximately $ million, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same. We may also increase or decrease the number of shares we are offering. An increase (decrease) of 1,000,000 in the number of shares we are offering would increase (decrease) the net proceeds to us from this offering, after deducting the underwriting discounts and commissions and estimated offering expenses payable by us, by approximately $ million, assuming the initial public offering price stays the same. An increase of 1,000,000 in the number of shares we are offering, together with a $1.00 increase in the assumed initial public offering price of $ per share (the midpoint of the estimated price range set forth on the cover page of this prospectus), would increase the net proceeds to us from this offering, after deducting the underwriting discounts and commissions and estimated offering expenses payable by us, by approximately $ million. A decrease of 1,000,000 in the number of shares we are offering, together with a $1.00 decrease in the assumed initial public offering price of $ per share (the midpoint of the estimated price range set forth on the cover page of this prospectus), would decrease the net proceeds to us from this offering, after deducting the underwriting discounts and commissions and estimated offering expenses payable by us, by approximately $ million. We do not expect that a change in the offering price or the number of shares by these amounts would have a material effect on our intended uses of the net proceeds from this offering, although it may impact the amount of time prior to which we may need to seek additional capital.
The principal purposes of this offering are to increase our financial flexibility, create a public market for our common stock and to facilitate our access to the public equity markets. We currently expect to use the net proceeds from this offering as follows:
| ● | approximately $ million for the commercialization of our Pure-Vu system; and | |
| ● | approximately $ million for research and development activities, including the continued development and enhancement of our Pure-Vu system. |
We expect to use the remainder of the net proceeds from this offering for working capital and other general corporate purposes, including the costs of operating as a public company. Although we currently anticipate that we will use the net proceeds from this offering as described above, there may be circumstances where a reallocation of funds is necessary. The amounts and timing of our actual expenditures will depend upon numerous factors, including our sales and marketing and commercialization efforts, demand for our products, our operating costs and the other factors described under “Risk Factors” in this prospectus. Accordingly, our management will have flexibility in applying the net proceeds from this offering. An investor will not have the opportunity to evaluate the economic, financial or other information on which we base our decisions on how to use the proceeds.
Although we may use a portion of the net proceeds of this offering for the acquisition or licensing, as the case may be, of additional technologies, other assets or businesses, or for other strategic investments or opportunities, we have no current understandings, agreements or commitments to do so.
Pending our use of the net proceeds from this offering, we intend to invest the net proceeds in a variety of capital preservation investments, including short-term, investment-grade, interest-bearing instruments and U.S. government securities.
| 29 |
We have never paid any cash dividends on our common stock. We anticipate that we will retain funds and future earnings to support operations and to finance the growth and development of our business. Therefore, we do not expect to pay cash dividends in the foreseeable future following this offering. Any future determination to pay dividends will be at the discretion of our board of directors and will depend on our financial condition, results of operations, capital requirements and other factors that our board of directors deems relevant. In addition, the terms of any future debt or credit financings may preclude us from paying dividends.
In addition, the ability of Opco, our direct wholly-owned operating subsidiary, to distribute dividends may be limited by Israeli law. The Israeli Companies Law, 1999, or the Israeli Companies Law, restricts Opco’s ability to declare dividends. Unless otherwise approved by a court, Opco can distribute dividends only from “profits” (as defined by the Israeli Companies Law), and only if there is no reasonable concern that the dividend distribution will prevent it from meeting its existing and foreseeable obligations as they become due. Dividends may be paid with the approval of a court, at a company’s request, provided that there is no reasonable concern that payment of the dividend will prevent the company from satisfying its current and foreseeable obligations, as they become due.
The payment of dividends by Opco to Holdings may be subject to Israeli withholding taxes.
| 30 |
The following table sets forth our capitalization as of June 30, 2017:
| ● | on an actual basis; | |
| ● | on a pro forma as-adjusted basis to reflect the issuance and sale by us of shares of our common stock in this offering at the assumed initial public offering price of $ per share (the midpoint of the estimated price range set forth on the cover page of this prospectus), after deducting underwriting discounts and commissions and estimated offering expenses payable by us and the receipt by us of the proceeds of such sale. |
You should read this information together with the sections entitled “Management’s Discussion and Analysis of Financial Condition and Results of Operations” as well as our financial statements and the related notes, which appear elsewhere in this prospectus.
| As of June 30, 2017 | ||||||||
| Actual | Pro Forma | |||||||
| (unaudited) | ||||||||
| (in thousands, except per share data) | ||||||||
| Cash and cash equivalents | $ | 12,940 | $ | |||||
| Other long-term liabilities | $ | 1,544 | $ | |||||
| Stockholders’ equity (deficit): | ||||||||
| Common stock, $0.0001 par value; 50,000,000 authorized, 10,489,066 issued and outstanding (actual), 13,822,399 issued and outstanding (pro forma) | 1 | |||||||
| Preferred series A stock, $0.0001 par value; 2,000,000 authorized, 1,581,128 issued and outstanding (actual and pro forma) | * | * | ||||||
| Preferred stock, $0.0001 par value; 8,000,000 authorized, 0 issued and outstanding (actual and pro forma) | - | - | ||||||
| Additional paid-in capital | 43,020 | |||||||
| Accumulated deficit | (32,182 | ) | ||||||
| Total shareholders’ equity | 10,839 | |||||||
| Total capitalization | $ | 12,383 | $ | |||||
* Represents amounts less than one thousand
A $1.00 increase (decrease) in the assumed initial public offering price of $ per share (the midpoint of the estimated price range set forth on the cover page of this prospectus) would increase (decrease) the amount of cash and cash equivalents, additional paid-in capital, total stockholders’ equity (deficit) and total capitalization on a pro forma as adjusted basis by approximately $ million, assuming the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting underwriting discounts and commissions and estimated offering expenses payable by us. Similarly, each increase (decrease) of one million shares offered by us would increase (decrease) cash and cash equivalents, total stockholders’ equity (deficit) and total capitalization on a pro forma as adjusted basis by approximately $ million, assuming the assumed initial public offering price of $ per share (the midpoint of the estimated price range set forth on the cover page of this prospectus) remains the same, and after deducting underwriting discounts and commissions and estimated offering expenses payable by us. A one million share increase in the number of shares offered by us together with a concomitant $1.00 increase in the assumed initial public offering price of $ per share (the midpoint of the estimated price range set forth on the cover page of this prospectus) would increase each of cash and cash equivalents and total stockholders’ (deficit) equity by approximately $ million after deducting underwriting discounts and commissions and any estimated offering expenses payable by us. Conversely, a one million share decrease in the number of shares offered by us together with a concomitant $1.00 decrease in the assumed initial public offering price of $ per share (the midpoint of the estimated price range set forth on the cover page of this prospectus) would decrease each of cash and cash equivalents and total stockholders’ (deficit) equity by approximately $ million after deducting underwriting discounts and commissions and any estimated offering expenses payable by us. The pro forma as adjusted information discussed above is illustrative only and will be adjusted based on the actual public offering price and other terms of this offering determined at pricing.
| 31 |
The number of shares of our common stock to be outstanding upon completion of this offering is based on 10,489,066 shares of our common stock outstanding as of June 30, 2017 and excludes:
| ● | 1,707,321 shares of our common stock issuable upon the exercise of outstanding stock options issued under our equity incentive plan as of June 30, 2017, at exercise prices ranging from $2.38 to $4.50 per share, following the modification of share-based payment awards on September 29, 2017; | |
| ● | 297,897 additional shares of our common stock reserved for future issuance under our 2016 Equity Incentive Plan as of June 30, 2017; | |
| ● | 1,581,128 shares of our common stock, issuable upon the conversion of Series A Convertible Preferred Stock issued in our 2017 Private Placement; | |
| ● | 907,237 shares of our common stock issuable upon the exercise of the Exchange Warrants (defined below, see “Business - Exchange of Convertible Note Warrants”), at an exercise price of $5.00 per share; | |
| ● | 403,632 shares of our common stock issuable upon the exercise of the Placement Agent Warrants (defined below, see “Business - 2017 Private Placement and Convertible Note Offering – Placement Agent Compensation”), at an exercise price of $5.00 per share; | |
| ● | 30,000 shares of our common stock issuable upon the exercise of a consultant warrant, at an exercise price of $8.00 per share; and | |
| ● | Any issuances of our securities after June 30, 2017. |
| 32 |
If you invest in our common stock in this offering, your ownership interest will be immediately diluted to the extent of the difference between the initial public offering price per share of our common stock and the pro forma as adjusted net tangible book value per share of our common stock immediately after this offering.
Our historical net tangible book value is the amount of our total tangible assets less our total liabilities. Net historical tangible book value per share is our historical net tangible book value divided by the number of shares of common stock outstanding as of June 30, 2017. Our pro forma net tangible book value as of June 30, 2017 was $ million, or $ per share of common stock. Our historical net tangible book value as of June 30, 2017 would have been approximately $ million, or $ per share of our pro forma outstanding common stock.
Pro forma as adjusted net book value is our pro forma net tangible book value, plus the effect of the sale of shares of our common stock in this offering at the assumed initial public offering price of $ per share (the midpoint of the estimated price range set forth on the cover page of this prospectus) and after deducting the underwriting discounts and commissions and estimated offering expenses payable by us. Our pro forma as adjusted net book value as of June 30, 2017 would have been approximately $ million, or $ per share. This amount represents an immediate increase in pro forma as adjusted net tangible book value of $ per share to our existing stockholders, and an immediate dilution of $ per share to new investors participating in this offering. Dilution per share to new investors is determined by subtracting pro forma as adjusted net tangible book value per share after this offering from the initial public offering price per share paid by new investors.
The following table illustrates this dilution on a per share basis:
| Assumed initial public offering price per share | $ | |||||||
| Historical net tangible book value (deficit) per share as of June 30, 2017 | $ | 1.03 | ||||||
| Pro forma increase in net tangible book value per share attributable to new investors | ||||||||
| Pro forma as adjusted net tangible book value per share, after giving effect to this offering | ||||||||
| Dilution of pro forma as adjusted net tangible book value per share to new investors | $ | |||||||
A $1.00 increase (decrease) in the assumed initial public offering price of $ per share (the midpoint of the estimated price range set forth on the cover page of this prospectus) would increase (decrease) the pro forma as adjusted net tangible book value, by $ per share and the dilution to new investors by $ per share, assuming the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same, and after deducting underwriting discounts and commissions and estimated expenses payable by us. An increase of one million shares offered by us would increase the pro forma as adjusted net tangible book value by $ per share and the dilution to new investors by $ per share, assuming the assumed initial public offering price of $ per share (the midpoint of the estimated price range set forth on the cover page of this prospectus) remains the same and after deducting underwriting discounts and commissions and estimated expenses payable by us. Similarly, a decrease of one million shares offered by us would decrease the pro forma as adjusted net tangible book value by $ per share and the dilution to new investors by $ per share, assuming the assumed initial public offering price of $ per share (the midpoint of the estimated price range set forth on the cover page of this prospectus) remains the same and after deducting underwriting discounts and commissions and estimated expenses payable by us. A one million share increase in the number of shares offered by us together with a concomitant $1.00 increase in the assumed initial public offering price of $ per share (the midpoint of the estimated price range set forth on the cover page of this prospectus) would increase the pro forma as adjusted net tangible book value by $ per share and the dilution to new investors by $ per share, after deducting underwriting discounts and commissions and any estimated offering expenses payable by us. Conversely, a one million share decrease in the number of shares offered by us together with a concomitant $1.00 decrease in the assumed initial public offering price of $ per share (the midpoint of the estimated price range set forth on the cover page of this prospectus) would decrease the pro forma as adjusted net tangible book value by $ per share and the dilution to new investors by $ per share, after deducting underwriting discounts and commissions and any estimated offering expenses payable by us.
| 33 |
If the underwriters exercise their over-allotment option in full, the pro forma as adjusted net tangible book value per share after giving effect to this offering would be $ per share, which amount represents an immediate increase in the pro forma as adjusted net tangible book value of $ per share of our common stock to existing stockholders and an immediate dilution in net tangible book value of $ per share of our common stock to new investors purchasing shares of common stock in this offering.
If any shares are issued upon conversion or exercise of outstanding Series A Convertible Preferred Stock, options or warrants, you will experience further dilution. The above discussion and table are based on 10,489,066 shares of our common stock outstanding as of June 30, 2017 and excludes:
| ● | 1,707,321 shares of our common stock issuable upon the exercise of outstanding stock options issued under our equity incentive plan as of June 30, 2017, at exercise prices ranging from $2.38 to $4.50 per share, following the modification of share-based payment awards on September 29, 2017; | |
| ● | 297,897 additional shares of our common stock reserved for future issuance under our 2016 Equity Incentive Plan as of June 30, 2017; | |
| ● | 1,581,128 shares of our common stock, issuable upon the conversion of Series A Convertible Preferred Stock issued in our 2017 Private Placement; | |
| ● | 907,237 shares of our common stock issuable upon the exercise of the Exchange Warrants (defined below, see “Business - Exchange of Convertible Note Warrants”), at an exercise price of $5.00 per share; | |
| ● | 403,632 shares of our common stock issuable upon the exercise of the Placement Agent Warrants (defined below, see “Business - 2017 Private Placement and Convertible Note Offering – Placement Agent Compensation”), at an exercise price of $5.00 per share; | |
| ● | 30,000 shares of our common stock issuable upon the exercise of a consultant warrant, at an exercise price of $8.00 per share; and | |
| ● | Any issuances of our securities after June 30, 2017. |
The following table summarizes, on the pro forma as adjusted basis described above as of June 30, 2017, the difference between the number of shares of common stock purchased from us, the total consideration paid to us and the average price per share paid to us by our existing stockholders and by new investors purchasing shares of common stock in this offering at the assumed initial public offering price of $ per share (the midpoint of the estimated price range set forth on the cover page of this prospectus) before the deduction of underwriting discounts and commissions and estimated offering expenses payable by us. Investors purchasing shares of our common stock in this offering will pay an average price per share higher than our existing stockholders paid.
| 34 |
| Shares Purchased | Total Consideration | Average
Price | ||||||||||||||||||
| Number | Percent | Amount | Percent | Per Share | ||||||||||||||||
| Existing stockholders before this offering | % | % | ||||||||||||||||||
| New Investors participating in this offering | % | % | ||||||||||||||||||
| Total | 100 | % | 100 | % | ||||||||||||||||
Each $1.00 increase (decrease) in the assumed initial public offering price of $ per share (the midpoint of the estimated price range set forth on the cover page of this prospectus) would increase (decrease) the total consideration paid by new investors by $ , and increase (decrease) the percentage of total consideration paid by new investors by approximately %, assuming that the number of shares offered by us, as listed on the cover page of this prospectus, remains the same.
Similarly, each increase (decrease) of one million shares in the number of shares of common stock offered by us would increase (decrease) the total consideration paid by new investors by $ million and increase (decrease) the percentage of total consideration paid by new investors by approximately % assuming that the assumed initial public offering price of $ per share (the midpoint of the estimated price range set forth on the cover page of this prospectus) price remains the same.
Certain of our existing stockholders, including stockholders affiliated with certain of our directors, have indicated an interest in purchasing up to an aggregate of approximately $ million in shares of our common stock in this offering at the initial public offering price. However, because indications of interest are not binding agreements or commitments to purchase, the underwriters could determine to sell more, less or no shares to any of these potential investors and any of these potential investors could determine to purchase more, less or no shares in this offering. The foregoing discussion and tables do not reflect any potential purchases by these potential investors or their affiliated entities.
After giving effect to the purchase of shares in this offering by these existing stockholders, assuming an initial public offering price of $ per share, the mid-point of the price range set forth on the cover page of this prospectus, our existing stockholders will hold % ( % if the underwriters exercise their over-allotment in full) of our common stock outstanding after this offering based on shares of our common stock outstanding as of June 30, 2017. The new investors purchasing the remaining shares in this offering will hold % ( % if the underwriters exercise their over-allotment in full) of our common stock outstanding after this offering.
To the extent that outstanding Series A Convertible Preferred Stock, options and warrants are converted or exercised, you will experience further dilution. In addition, we may choose to raise additional capital due to market conditions or strategic considerations even if we believe we have sufficient funds for our current or future operating plans. To the extent that additional capital is raised through the sale of equity or convertible debt securities, the issuance of these securities may result in further dilution to new investors participating in this offering.
| 35 |
MANAGEMENT’S
DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
Prospective investors should read the following discussion and analysis of our financial condition and results of operations together with our consolidated financial statements and the related notes and other financial information included elsewhere in this prospectus. Some of the information contained in this discussion and analysis or set forth elsewhere in this prospectus, including information with respect to our plans and strategy for our business, includes forward-looking statements that involve risks and uncertainties. See “Cautionary Note Regarding Forward-Looking Statements.” You should review the “Risk Factors” section of this prospectus for a discussion of important factors that could cause actual results to differ materially from the results described in or implied by the forward-looking statements contained in the following discussion and analysis.
Overview
We have developed a single-use medical device system (the “Pure-Vu system”), cleared by the United States Food and Drug Administration (the “FDA”), that cleanses the colon during a colonoscopy procedure thereby reducing the dependency on unpleasant and time consuming pre-procedural colonoscopy preparation regimens to ensure clear visualization of the colon mucosa which is critical to procedural success. The Pure-Vu system has been designed to integrate with standard colonoscopes to enable cleaning during the procedure while preserving standard procedural workflow and techniques. We believe the Pure-Vu system has the potential to improve procedure quality, clinical outcomes and patient satisfaction while simultaneously lowering costs and improving margins for hospitals and increasing revenues and improving margins for outpatient colonoscopy clinics. Our Pure-Vu system and the procedure to cleanse the colon in preparation for colonoscopy are not currently reimbursable through private or governmental third-party payors in any country, but we do intend to seek reimbursement through private or governmental third-party payors in the future. To date, as part of our limited pilot launch, we have focused on collecting clinical data on the use of the Pure-Vu system. We do not expect to generate significant revenue from product sales unless and until we expand our commercialization efforts.
Our business was founded within the New Generation Technology (“NGT”) incubator based in Nazareth, Israel in 2008 to develop innovative endoscopy technologies, and in 2011, the business was spun out into a new company focused exclusively on the development of the Pure-Vu system. We initiated preclinical testing in 2011 and started clinical testing of the first prototype version of the Pure-Vu system in Europe in late 2012. In clinical studies performed in Europe and Israel from 2012 through the second quarter of 2017, the Pure-Vu system and earlier prototype versions have demonstrated effective cleaning in over 175 patients that followed a significantly reduced pre-procedural colonoscopy preparation regimen, as compared to current prep regimens.
Financial Operations Overview
We are a development stage company and have not generated any significant revenues from the sale of products. We have never been profitable and our accumulated deficit as of June 30, 2017 was approximately $32.2 million. Our net loss for the six months ended June 30, 2017 and 2016 was approximately $6.26 million and $3.67 million, respectively. Our net loss for the years ended December 31, 2016 and 2015 were approximately $8.0 million and $6.0 million. We expect to incur significant expenses and increasing operating losses for the foreseeable future. We expect our expenses to increase significantly in connection with our ongoing activities to commercialize and market the Pure-Vu system. Furthermore, we expect to incur additional costs associated with operating as a public company. Accordingly, we will need additional financing to support our continuing operations. We will seek to fund our operations through public or private equity or debt financings or other sources, which may include collaborations with third parties. Adequate additional financing may not be available to us on acceptable terms, or at all. Our failure to raise capital as and when needed would have a negative impact on our financial condition and our ability to pursue our business strategy. We will need to generate significant revenues to achieve profitability, and we may never do so.
| 36 |
We expect to continue to incur significant expenses and increasing operating losses for at least the next several years. We expect our expenses will increase substantially in 2017 and in the future in connection with our ongoing activities, as we:
| ● | conduct a limited pilot launch through 2018 to refine how the Pure-Vu system integrates into the workflow of both the out-patient and in-patient settings; | |
| ● | manufacture the Pure-Vu system in our facility in Israel to support the initial pilot launch in the U.S.; | |
| ● | contract with third parties to transfer and scale up the manufacture of the workstation and the disposable portion of Pure-Vu system; | |
| ● | develop a second generation system to improve user interface, optimize ease of use and reduce the cost structure; | |
| ● | raise sufficient funds in the capital market to effectuate our business plan, including commercialization of our Pure-Vu system; and | |
| ● | operate as a public company. |
Critical Accounting Policies and Estimates
Our consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America. The preparation of these financial statements requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period.
On an ongoing basis, we evaluate our estimates and judgments for all assets and liabilities, including those related to fair value calculations for equity securities, assessing contingent liabilities, establishing valuation allowances for deferred taxes, and the recovery of deferred costs. We base our estimates and judgments on historical experience, current economic and industry conditions and on various other factors that are believed to be reasonable under the circumstances. This forms the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
We believe that full consideration has been given to all relevant circumstances that we may be subject to, and the consolidated financial statements accurately reflect our best estimate of the results of operations, financial position and cash flows for the periods presented.
Revenue
To date, as part of our limited launch, we have generated some revenue from the sales of products. We do not expect to generate significant revenue from product sales unless and until we expand our commercialization efforts for the Pure-Vu system, which we expect will take a number of years and is subject to significant uncertainty.
Research and Development
We incurred expenses of approximately $1.7 million and $1.8 million, respectively, during the six months ended June 30, 2017 and 2016 for research and development activities. We incurred net expenses of approximately $3.1 million and $3.2 million, respectively, during the years ended December 31, 2016 and 2015 for research and development activities. These expenses include cash and non-cash expenses relating to the development of our development and clinical programs for the Pure-Vu system. We have research and development capabilities in electrical and mechanical engineering with laboratories in our facility in Israel for development and prototyping, and electronics design and testing. We also use consultants and third party design houses to complement our internal capabilities.
Sales and Marketing
We incurred expenses of approximately $1.0 million and $0.3 million, respectively, during the six months ended June 30, 2017 and 2016 for sales and marketing activities. We incurred expenses of approximately $1.0 million and $0.4 million, respectively, during the years ended December 31, 2016 and 2015 for sales and marketing activities. These expenses include cash and non-cash expenses relating to the development of our sales and marketing infrastructure for the Pure-Vu system. We have hired limited sales and marketing personnel in the US as part of our pilot launch to develop our policies and procedures, as well as to spearhead the pilot phase of the company’s market penetration.
| 37 |
General and Administrative Expenses
We incurred expenses of approximately $2.5 million and $0.8 million, respectively, during the six months ended June 30, 2017 and 2016 for general and administrative activities. We incurred expenses of approximately $1.9 million and $1.8 million, respectively, during the years ended December 31, 2016 and 2015 for general and administrative activities. General and administrative expenses consist primarily of payroll and professional services. Other general and administrative expenses include accounting and legal services and expenses associated with obtaining and maintaining patents. We anticipate that our general and administrative expenses will increase significantly during 2017 and in the future as we increase our headcount to support our continued development and the commercialization of our Pure-Vu system. We also anticipate increased expenses related to audit, legal, regulatory, and tax-related services associated with maintaining compliance with exchange listing and SEC requirements, director and officer insurance premiums, and investor relations costs associated with being a public company. Additionally, commencing in 2017, we began to compensate our outside directors.
Stock-Based Compensation
In 2016, we adopted the 2016 Equity Incentive Plan. No new equity awards were issued pursuant to the 2016 Equity Incentive Plan as of December 31, 2016, however all of the issued and outstanding options to purchase or otherwise acquire shares of Opco capital stock that were outstanding and unexercised as of immediately prior to the Initial Closing were substituted for 125,730 options to acquire shares of our common stock pursuant to our 2016 Equity Incentive Plan on the date of the Initial Closing. Equity awards, including options to purchase 1,726,770 shares of our common stock at an exercise price of $4.50 per share, following the modification of such share-based payment awards on September 29, 2017, and unrestricted stock awards for 5,000 shares of our common stock were issued pursuant to the 2016 Equity Incentive Plan from January 1, 2017 through June 30, 2017. The fair value of the equity awards granted was $1,383,996. Compensation expense for equity awards is recognized over the period of service, generally the vesting period.
Emerging Growth Company Status
Under Section 107(b) of the JOBS Act, emerging growth companies can delay adopting new or revised accounting standards until such time as those standards apply to private companies. We have irrevocably elected not to avail ourselves of this exemption from new or revised accounting standards and, therefore, we will be subject to the same new or revised accounting standards as other public companies that are not emerging growth companies.
Results of Operations
Comparison of Six Months Ended June 30, 2017 and 2016
Research and Development. Research and development expenses for the six months ended June 30, 2017 totaled $1.7 million, a decrease of $0.1 million, or 5.5%, from the $1.8 million recorded for the six months ended June 30, 2016. The $0.1 million decrease was primarily attributed to an increase of $0.36 million in research and development material cost, $0.31 million increase in salaries and wages, $.08 million increase in stock compensation and other research and development cost which was offset with a $0.9 million decrease in payments to consultants and subcontractors.
Sales and Marketing. Sales and marketing expense for the six months ended June 30, 2017 totaled $1.0 million, an increase of $0.7 million, or 233%, from the $0.3 million recorded for the six months ended June 30, 2016. The $0.7 million increase was primarily attributed to an increase of $0.56 million in salaries and wages, $.08 million increase in travel related cost and $.06 million increase in stock compensation and other sales and marketing cost.
General and Administrative. General and administrative expense for the six months ended June 30, 2017 totaled $2.5 million, an increase of $1.7 million, or 212%, from the $0.8 million recorded for the six months ended June 30, 2016. The $1.7 million increase was primarily attributed to an increase of $0.26 million in salaries and wages, $0.17 million increase in rent and office related expenses, $0.73 million increase in professional and consulting fees, $0.52 million increase in stock compensation and other general and administrative cost.
| 38 |
Comparison of Year Ended December 31, 2016 and 2015
Research and Development. Research and development expenses for the year ended December 31, 2016 totaled $3.1 million, a slight decrease of $0.1 million, or 3.1%, from the $3.2 million recorded for the year ended December 31, 2015.
Sales and Marketing. Sales and marketing expense for the year ended December 31, 2016 totaled $1.0 million, an increase of $0.6 million, or 150%, over the $0.4 million recorded for the year ended December 31, 2015.
General and Administrative. General and administrative expense for the year ended December 31, 2016 totaled $1.9 million, a slight increase of $0.1 million, or 5.6%, over the $1.8 million recorded for the year ended December 31, 2015.
Liquidity and Capital Resources
Since inception, we have experienced negative cash flows from operations. We have financed our operations primarily through sales of equity-related securities and convertible notes. At June 30, 2017, our accumulated deficit since inception was approximately $32.2 million.
At June 30, 2017, we had total current assets of approximately $14.0 million and current liabilities of approximately $2.4 million resulting in working capital of $11.6 million. At June 30, 2017, we had total assets of approximately $14.7 million and total liabilities of approximately $3.9 million, resulting in a stockholders’ equity of $10.8 million.
Net cash used in operating activities for the six months ended June 30, 2017 was approximately $4.7 million, which includes cash used from a net loss of approximately $6.3 million and $0.8 million of cash used from an increase in accounts receivable, inventory, and other current assets. This was partially offset by an increase in accounts payable and other current liabilities of $1.6 million and by non-cash items included in the net loss of $0.8 million in stock-based compensation and revaluation of convertible notes and other long-term liabilities.
Net cash used in operating activities for the twelve months ended December 31, 2016 was approximately $6.1 million, which includes cash used from a net loss of approximately $8.0 million, cash used from a decrease in accounts payable expenses totaling $0.4 million and $0.2 million of cash used from an increase in inventory and other current assets. This was offset by non-cash items included in the net loss of $2.0 million for interest and revaluation of convertible notes and a $0.4 million increase in other payables.
Net cash provided from financing activities for the six months ended June 30, 2017 totaled approximately $6.4 million from the issuance of our common stock and preferred stock in the 2017 Private Placement net of issuance costs.
Net cash provided from financing activities for the twelve months ended December 31, 2016 totaled approximately $16.5 million from the issuance of our common stock and preferred stock in the 2017 Private Placement. A summary table of the net cash proceeds received from convertible notes and the sale of equity in the 2017 Private Placement is as follows:
| Net proceeds from issuance of convertible notes | $ | 9,606 | ||
| Net proceeds from issuance of equity - common and preferred | $ | 6,878 | ||
| Total Net proceeds | $ | 16,484 |
Net cash used in investing activities was $0.5 million for the six months ended June 30, 2017.
Net cash used in investing activities was only $6,000 for the twelve months ended December 31, 2016.
| 39 |
At June 30, 2017, we had no debt outstanding.
At June 30, 2017, we had a cash and cash equivalents balance of approximately $12.9 million. We have included a going concern provision in our financial statements as of June 30, 2017, expressing substantial doubt that we can continue as an ongoing business for the next twelve months without additional financing. However, we believe our current cash and cash equivalents, together with the net proceeds from this offering, will be sufficient to meet our anticipated cash requirements over at least the next 18 months. We will need to raise significant additional capital to fund the commercialization of our Pure-Vu system. The source, timing and availability of any future financing will depend principally upon market conditions, and, more specifically, on the progress of our U.S. market entry strategy. Funding may not be available when needed, at all, or on terms acceptable to us. Lack of necessary funds may require us, among other things, to delay, scale back or eliminate our market entry strategy.
Contractual Obligations and Commitments
We may enter into contracts in the normal course of business with suppliers and other vendors for operating purposes. These contracts generally provide for termination on notice, and therefore we believe that our non-cancelable obligations under these agreements are not material. As of June 30, 2017, we had no material Contractual Obligations or Commitments that will affect our future liquidity.
On January 1, 2015, we entered into a five year lease for a facility with 7,732 square feet of space in Tirat Carmel, Israel. Annual rent is $82,000 per year.
On April 13, 2017, we entered into a lease for a facility in Fort Lauderdale, Florida, which we intend to begin occupying in October 2017. The facility will be initially 4,554 square feet, which will increase to 6,390 square feet by the second year of the lease. The term will run for seven years and two months from the date we begin to occupancy the facility. Annual base rent is initially $156,555 per year, subject to annual increases of 2.75%.
Off-Balance Sheet Arrangements
We did not have during the periods presented, and we do not currently have, any off-balance sheet arrangements, as defined under SEC rules, such as relationships with unconsolidated entities or financial partnerships, which are often referred to as structured finance or special purpose entities, established for the purpose of facilitating financing transactions that are not required to be reflected on our balance sheets.
Quantitative and Qualitative Disclosures about Market Risk
Our exposure to market risk is limited to our cash and cash equivalents, all of which have maturities of three months or less. The primary objectives of our investment activities are to preserve principal, provide liquidity and maximize income without significantly increasing risk. Our primary exposure to market risk is interest income sensitivity, which is affected by changes in the general level of U.S. interest rates. However, because of the short-term nature of the instruments in our portfolio, a sudden change in market interest rates would not be expected to have a material impact on our financial condition and/or results of operation. We do not have any foreign currency or other derivative financial instruments.
| 40 |
Overview
We have developed a single-use medical device system (the “Pure-Vu system”), cleared by the United States Food and Drug Administration (the “FDA”), that cleanses the colon during a colonoscopy procedure thereby reducing the dependency on unpleasant and time consuming pre-procedural colonoscopy preparation regimens to ensure clear visualization of the colon mucosa which is critical to procedural success. The Pure-Vu system has been designed to integrate with standard colonoscopes to enable cleaning during the procedure while preserving standard procedural workflow and techniques. We believe the Pure-Vu system has the potential to improve procedure quality, clinical outcomes and patient satisfaction while simultaneously lowering costs and improving margins for hospitals and increasing revenues and improving margins for outpatient colonoscopy clinics. Our Pure-Vu system and the procedure to cleanse the colon in preparation for colonoscopy are not currently reimbursable through private or governmental third-party payors in any country, but we do intend to seek reimbursement through private or governmental third-party payors in the future. To date, as part of our limited pilot launch, we have focused on collecting clinical data on the use of the Pure-Vu system. We do not expect to generate significant revenue from product sales unless and until we expand our commercialization efforts.
Our business was founded within the New Generation Technology (“NGT”) incubator based in Nazareth, Israel in 2008 to develop innovative endoscopy technologies, and in 2011, the business was spun out into a new company focused exclusively on the development of the Pure-Vu system. We initiated preclinical testing in 2011 and started clinical testing of the first prototype version of the Pure-Vu system in Europe in late 2012. In clinical studies performed in Europe and Israel from 2012 through the second quarter of 2017, the Pure-Vu system and earlier prototype versions have demonstrated effective cleaning in over 175 patients that followed a significantly reduced pre-procedural colonoscopy preparation regimen, as compared to current prep regimens.
Market Overview
Colonoscopies are one of the most frequently performed medical procedures with over 15 million colonoscopies performed in the U.S. every year and close to 30 million worldwide. In the U.S., approximately 90% of the 15 million colonoscopies are performed as out-patients (13.5 million) at an ambulatory endoscopy center, or AEC, and or hospital out-patient departments, or HOPD, and 10% as in-patients (1.5 million) in hospitals. Approximately 60% of colonoscopies are performed to detect and prevent colorectal cancer, or CRC, which is the second leading cause of cancer-related deaths in the U.S. with approximately 140,000 new cases diagnosed and 50,000 deaths every year. Over the past few decades, CRC has been demonstrated to be one of the most preventable cancers through the use of colonoscopies, which is the gold standard for CRC screening. The remaining 40% of colonoscopies are performed to help diagnose and treat other gastrointestinal, or GI, conditions including irritable bowel syndrome, or IBS, inflammatory bowel disease, or IBD, anemia and lower GI bleeding.
Despite the pervasiveness and effectiveness of colonoscopy, a key ongoing clinical challenge of the procedure is that patients are required to undergo a potent pre-procedure bowel preparation regimen to try to ensure that the colon is fully cleansed to enable clear visualization of the tissue. The regimens can be highly disruptive and uncomfortable for many patients. Further, it has been widely reported that approximately 23% of out-patients and 45% of in-patients present with inadequately prepped colons, resulting in a number of colonoscopies that yield poor diagnostic accuracy or failed colonoscopies that must be repeated. Another key problem is that approximately 35% of eligible patients are not current with their CRC screening in the U.S. based on current guidelines. One of the primary reasons patients fail to get a screening colonoscopy or to return for follow-up procedures is the fear or dislike of the potent and unpleasant preparation required prior to the procedure.
Successful bowel preparation is one of the most important factors in delivering a thorough, high quality exam and is well documented to have a direct impact on the adenoma detection rate (the rate of detecting pre-cancer anomalies in the colon tissue). The preparation regimen typically requires patients to be on a liquid diet for over 24 hours, drink up to four liters of a purgative, spend up to 12 hours prior to the exam periodically going to the bathroom to empty their bowels, and disrupting their daily activities, which could include missing work or other activities. The cleansing process is well known to be inconvenient and uncomfortable for many patients resulting in up to twenty percent (20%) of patients arriving for their colonoscopy inadequately cleansed. An inadequately prepared colon can impact the diagnostic accuracy of the procedure and can lead to procedures having to be repeated earlier than the medical guidelines advise or can lead to failed procedures especially in the in-patient setting. Rescheduling the procedure is inconvenient to the patient (and many patients fail to come for their follow-up), creates inefficiencies in the provider’s workflow, and increases the length of hospital stay for the in-patient, each of which results in increased healthcare costs.
| 41 |
Our Pure-Vu Solution
To address this unmet need, we have developed our FDA-cleared Pure-Vu system, which readily integrates with existing colonoscopes to cleanse poorly prepped colons during the colonoscopy procedure, thereby greatly reducing the dependency on conventional pre-procedural bowel prep regimens to get a clear visualization of colon tissue. Our system consists of a workstation controller and a single-use, disposable sleeve that fits over most standard-size commercial colonoscopes. Together with the colonoscope, the Pure-Vu system performs rapid, effective and efficient intra-procedural cleaning without compromising procedural workflow and techniques. The over-sleeve has an umbilical section that connects to a cartridge that mounts to the workstation and serves as the interface between the disposable over-sleeve and the workstation. The workstation, through a series of peristaltic pumps activated by foot pedals, delivers safe and highly effective irrigation medium of air and water that creates a pulsed vortex inside the colon to break up fecal matter while simultaneously evacuating the colon content into waste receptacles already used in a standard colonoscopy procedure. The proprietary evacuation system in the device has sensors built in that can detect the formation of a blockage and automatically clear it allowing the physician to remove significant debris from the patient. The Pure-Vu has been clinically demonstrated to be capable of cleaning poorly prepared colons in minutes. We are building an extensive intellectual property portfolio designed to protect key aspects of the system, including the pulsed vortex irrigation and auto-purge functions.
The Pure-Vu System

In the out-patient setting, the Pure-Vu system creates the opportunity to improve patient satisfaction, enhance diagnostic quality while providing a new source of revenue for out-patient colonoscopy clinics such as AECs, or HOPDs. Additionally, in the in-patient hospital setting, the Pure-Vu system creates the opportunity to improve the time to prepare for and complete a successful colonoscopy and thereby reduce the patient’s length of stay, providing significant cost savings to the hospital which typically receives a fixed reimbursement under a diagnostic related group (“DRG”) code and does not receive any additional reimbursement for delays related to bowel preparation challenges.
Out-patient Opportunity: improving patient experience and reducing repeat procedures
The largest commercial market opportunity for us is in the out-patient setting offering patients an alternative to the arduous experience of having to drink large volumes of purgatives that result in significant discomfort, multiple visits to the bathroom over a many-hour period and disruption of daily activities. Market research conducted by The Nova Group, which was sponsored by us, indicates that 83% of patients are willing to pay for this type of technology and 29% are willing to consider paying up to $350 out-of-pocket for the ability to follow a “less-prep” regimen. As the potential out-of-pocket cost of a Pure-Vu examination is reduced, the percentage of patients willing to consider paying increases dramatically. With increasing pressure on physician and facility reimbursement, most providers are incorporating ancillary services into their practices to supplement their revenue and increase profit. Incorporation of the Pure-Vu system as an ancillary product into an out-patient GI practice is expected to provide an additional source of revenue and profit as well as help to differentiate the GI practice in an increasingly competitive marketplace. By offering a solution to those patients who either cannot tolerate the challenging preparation or desire a more tolerable prep, we believe the GI practice can increase their market share and improve patient satisfaction, a key quality metric being measured by payers. The Pure-Vu system can also facilitate late afternoon and early evening procedures for those patients wishing to avoid disruption in their daily activities. Finally, with the Pure-Vu system, the prep may no longer be as significant of a deterrent to receiving a colonoscopy, potentially increasing compliance to screening and ultimately increasing the early detection of CRC.
| 42 |
The Pure-Vu system also has the potential to reduce the number of early repeat procedures due to inadequate preparation and therefore reduce healthcare costs. Based on published literature in several peer reviewed journals from 2010 to 2015 and surveys of physicians conducted in 2015, approximately 20% of patients can have an inadequate preparation, that may lead to repeat procedures earlier than the medical guidelines suggest and decrease the adenoma detection rate negatively effecting the quality of the exam. By giving the physician the ability to effectively cleanse the colon intra-procedurally, the Pure-Vu system provides the ability to turn a fair or poor preparation into an optimal preparation and achieve a high-quality colonoscopy.
In-patient Opportunity: improving efficiencies and shortening time to complete a successful colonoscopy
In-patient colonoscopy is usually performed to diagnose the source of various gastrointestinal conditions such as lower GI bleeding or bowel pain. For an in-patient hospital stay, the Centers for Medicare and Medicaid Services, or CMS, uses a prospective payment system, or PPS, based upon diagnostic related groups, or DRG, to pay for hospital services with the goal of encouraging providers to minimize their costs. The DRG assignment is influenced by a combination of factors such as a patient’s sex, diagnosis at the time of discharge and procedures performed. Based on patient specific information, all hospital expenses for their care during an inpatient stay are packaged and assigned to one of over 500 MS-DRGs. According to Decision Driver Analytics, a reimbursement consulting agency, when a colonoscopy is performed as the primary procedure (no other procedures or complicating diagnosis), DRGs 395, 394 or 393 would apply which pay between $3,861 (without complications or major comorbidities) and $9,421 (with major complications and comorbidities). The cost for just one night in the hospital averages $1,800, so reducing the length of stay can save the hospital significant expense.
An in-patient colonoscopy is more problematic than an out-patient procedure due primarily to poorer quality bowel prep which can lead to lower rates of successful completion of the procedure and a higher frequency of repeat procedures. Inadequate bowel prep rates have been reported in the literature as high as 45% for the in-patient setting. Managing these patients is a challenge often requiring significant healthcare provider resources to administer and monitor the prep. The poor bowel prep can be due to the patient’s condition as a more fragile patient population may be unable to tolerate the significant volume of fluid required, and the clear liquid diet required, to cleanse the colon. With these patients, a high volume of purgative can also lead to electrolyte imbalances. The Pure-Vu system can shorten the time to successfully complete a colonoscopy by streamlining the process with effective and safe intra-procedural cleaning thus reducing healthcare costs.
Pre-Clinical and Clinical Data & Safety
In clinical studies performed in Europe and Israel, the Pure-Vu system and earlier prototype versions have demonstrated effective cleaning in over 175 patients receiving a reduced prep regimen. The first 83 patients used three different versions of the system. The prep regimen used in these patients varied from taking a 50% dose of the standard PEG based prep to as little as taking 20mg of over-the-counter Dulcolax (bisocodyl). More recently, the commercial version of the Pure-Vu system was used in two multi-center clinical studies. The first study involved 49 patients and was completed in the second quarter of 2016. The second study was completed in June 2017 and involved 47 patients. Patients in these studies had a restricted diet for 18-24 hours and received a split dose of 20mg of over-the-counter Dulcolax (bisocodyl). Patients did not take any liquid purgative traditionally prescribed for bowel preparation. The clinical data showing performance of the Pure-Vu system in the first of these studies using the Boston Bowel Preparation Score, the most commonly used method for evaluating the quality of bowel preparation, is shown below. The clinical results from the 2016 study were presented at United European Gastroenterology Week (UEGW) in October 2016. The clinical results from the 2017 study are expected to be presented at the UEGW in October 2017, showing similar results.
| 43 |
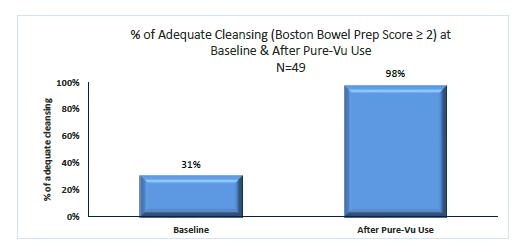
In addition, pre-clinical experience in a porcine animal model, which was used in the FDA submission, was also presented at Digestive Disease Week (“DDW”) in May 2016. In this study the animals were fasted from normal feed following the Day -2 morning meal. On the afternoon of Day -2, the animals received a standard three (3) liters of PEG-based colon preparation agent (Golytely). This data is presented below.
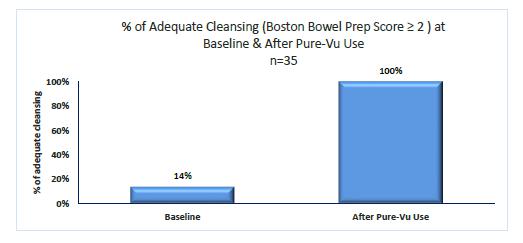
We are planning to initiate post-market surveillance and clinical study programs that may involve registries, investigator sponsored studies and company sponsored studies to drive clinical and health economic data, to support product development, enhance our marketing efforts and facilitate new indications. The first of these studies is expected to be initiated in the fourth quarter of 2017 and continue through 2018.
The Pure-Vu system is currently indicated to connect to standard colonoscopes to facilitate intra-procedural cleaning of a poorly prepared colon by irrigating or cleaning the colon and evacuating the irrigation fluid (water), feces and other bodily fluids and matter. We do not currently promote a particular prep regiment as this is left up to the discretion of the physician since our current indication does not reference any preparation protocol. We will look to expand the Pure-Vu system indication to allow us to actively promote minimal prep capabilities directly to patients. We plan to initiate a clinical trial in 2018 that should facilitate approval of expanded labeling in 2019.
| 44 |
We filed for a CE Mark in Europe in the second quarter of 2017 and anticipate CE Mark clearance in the fourth quarter of 2017. We intend to establish relationships with strategic partners for Europe, Japan, China and other key markets outside the US (“OUS”) to support the regulatory process and market entry. We anticipate entering OUS markets with our second-generation Pure-Vu system during the second half of 2019. We filed a special 510(k) with the FDA in the third quarter of 2017 to adjust our labeling to simplify the process of removing the Pure-Vu system from a colonoscope and to support minor enhancements to the manufacturing of the system.
Intellectual Property
Our IP position comprises a highly innovative portfolio covering technologies rooted in systems and methods for cleaning body cavities with or without the use of an endoscope. Currently we have three issued patents and 27 (13 in the US) pending patent applications in various regions of the world with a focus on the US, EU and Japan. Our earliest patent application filing dates go back to October 2007. We have also recently received notice of allowance for Motus GI and for Pure-Vu trademarks from the USPTO. We are pursuing these marks in the EU as well.
Our issued patents cover an endoscopic device insertable into a body cavity and movable in a predetermined direction and method of moving the endoscopic device in a body cavity and expire October 2026. Our patent application portfolio focuses on cleaning body cavities in a safe and efficient manner, insertion and movement and steering of an endoscopic device within the body cavity in a predetermined direction, coordinated positioning of an endoscope with a suction device and cleaning systems with automatic self-purging features. Our applications cover critical aspects of our system that we believe are key to effectively and efficiently clean the colon or other cavities in the body. These areas include cleansing jet methodologies, sensing and control of evacuation to avoid clogging, designs for easy attachment to endoscopes and cleaning segments under water.
Our commercial success depends in part on our ability to obtain and maintain patent and other proprietary protection for Pure-Vu and to operate without infringing the proprietary right of others and to prevent others from infringing our proprietary rights. We strive to protect our intellectual property through a combination of patents, and trademarks as well as through the confidentiality provisions in our contracts. With respect to Pure-Vu, we endeavor to obtain and maintain patent protection in the United States and internationally on all patentable aspects of the system. We cannot be sure that the patents will be granted with respect to any patent applications we may own or license in the future, nor can we be sure that our existing patents or any patents we may own or license in the future will be useful in protecting our technology.
In addition to patents, we rely on trade secrets and know-how to develop and maintain our competitive position. For example, significant aspects of our proprietary technology platform are based on unpatented trade secrets and know-how. Trade secrets and know-how can be difficult to protect. We seek to protect our proprietary technology and processes, in part, by confidentiality agreements and invention assignment agreements with our employees, consultants, scientific advisors, contractors and commercial partners. These agreements are designed to protect our proprietary information and, in the case of the invention assignment agreements, to grant us ownership of technologies that are developed through a relationship with a third party. We also seek to preserve the integrity and confidentiality of our data and trade secrets by maintaining physical security of our premises and physical and electronic security of our information technology systems. While we have confidence in these individuals, organizations and systems, agreements or security measures may be breached, and we may not have adequate remedies for any breach. In addition, our trade secrets may otherwise become known or be independently discovered by competitors. To the extent that our contractors use intellectual property owned by others in their work for us, disputes may arise as to the rights in related or resulting know-how and inventions.
We also plan to seek trademark protection in the United States and outside of the United States where available and when appropriate. We intend to use these registered marks in connection with our pharmaceutical research and development as well as our product candidates.
Our Formation
In connection with our formation in September 2016, we sold an aggregate of 1,650,000 shares of our common stock for an aggregate of $82,500 ($0.05 per share).
| 45 |
The Share Exchange Transaction
Effective on December 1, 2016, Opco, and the Opco Stockholders, entered into the Share Exchange Agreement with us. Pursuant to the terms of the Share Exchange Agreement, as a condition of and contemporaneously with the Initial Closing of the 2017 Private Placement, the Opco Stockholders sold to us, and we acquired, all of the issued and outstanding shares of capital stock of Opco and Opco became our direct wholly-owned subsidiary. Pursuant to the Share Exchange Transaction (i) the Opco Stockholders received an aggregate of 4,000,000 shares of our common stock in exchange for all of the issued and outstanding shares of capital stock of Opco, (ii) the Convertible Notes and the Convertible Note Warrants (as defined below) were exchanged for our securities, as described below, and (iii) all of the issued and outstanding options to purchase or otherwise acquire shares of Opco capital stock that were outstanding and unexercised as of immediately prior to the Initial Closing were substituted for 125,730 options to acquire shares of our common stock pursuant to our 2016 Equity Incentive Plan at exercise prices ranging from $2.38 to $2.52 (see “Executive Compensation—2016 Equity Incentive Plan”).
The Share Exchange Transaction was treated as a recapitalization of Opco for financial accounting purposes and the historical financial statements of Opco are our financial statements as a result of the Share Exchange Transaction.
2017 Private Placement and Exchange of Convertible Notes
In connection with the 2017 Private Placement we issued an aggregate of 3,080,671 Units for gross proceeds of approximately $15,400,000, comprised of an aggregate of 2,310,503 shares of our common stock and 770,168 shares of Series A Convertible Preferred Stock to investors in the 2017 Private Placement. Certain related parties participated in the 2017 Private Placement, see “Certain Relationships and Related Party Transactions - Convertible Note, Convertible Warrant, and 2017 Private Placement - Related Party Participation.”
In addition, from June 2015 through November 2016, pursuant to the terms of the CNA, Opco issued Convertible Notes in an aggregate amount of $14,596,683 (inclusive of accrued interest through December 22, 2016, the date of the Initial Closing) to certain investors, including related parties of us and Opco (see “Certain Relationships and Related Party Transactions - Convertible Note, Convertible Warrant, and 2017 Private Placement - Related Party Participation”) As part of the 2017 Private Placement, at the Initial Closing, the Convertible Holders exchanged their Convertible Notes, together with accrued and unpaid interest thereon at a rate of 10% per annum, for Units of the 2017 Private Placement, at a conversion price of $4.50 per Unit. As a result, upon consummation of the Initial Closing, the Convertible Notes were exchanged for an aggregate of 3,243,744 Units representing (i) 2,432,808 shares of our common stock (inclusive of shares of our common stock resulting from the accrued and unpaid interest of the Convertible Notes through the date of the Initial Closing) and (ii) 810,960 shares of our Series A Convertible Preferred Stock (inclusive of shares of Series A Convertible Preferred Stock resulting from the accrued and unpaid interest of the Convertible Notes through the date of the Initial Closing).
Exchange of Convertible Note Warrants
In connection with, and pursuant to the terms of, the CNA, each Convertible Holder also received a seven (7) year warrant (the “Convertible Note Warrants”) to purchase preferred A shares of Opco (the “Preferred A Shares of Opco”), nominal value in Israeli New Shekel (“NIS”) 0.01 per share, with an exercise price per share of $1.00 (the “Convertible Note Warrant Exercise Price”). Each Convertible Note Warrant entitled the holder to purchase that number of shares of Preferred A Shares of Opco equal to thirty-three percent (33%) of the principal amount of the Convertible Note in connection with which it was issued. Convertible Note Warrants to purchase an aggregate 4,536,186 shares of Preferred A Shares of Opco with an exercise price of $1.00 were issued in connection with the CNA. Certain related parties held Convertible Note Warrants pursuant to the CNA, see “Certain Relationships and Related Party Transactions - Convertible Note, Convertible Warrant, and 2017 Private Placement - Related Party Participation.” At the Initial Closing, the holders of the Convertible Note Warrants exchanged their Convertible Note Warrants for five (5) year warrants (the “Exchange Warrants”) to purchase an aggregate 907,237 shares of our common stock at an exercise price of $5.00 per share, such amount being equal to thirty-three percent (33%) of the principal amount of the Convertible Notes divided by $5.00.
| 46 |
2017 Private Placement and Convertible Note Offering – Placement Agent Compensation
Pursuant to a Finders Agreement entered into as of October 14, 2016 (the “Finders Agreement”), the Placement Agent assisted Opco with the offering of certain of the Convertible Notes by introducing individuals and entities (each a “Target”) interested in investing in Opco. In connection with those Convertible Notes purchased by Targets, and pursuant to the terms of the Finders Agreement, Opco paid the Placement Agent an aggregate fee of $552,500, consisting of (i) a cash fee in the aggregate amount of $425,000, such amount equal to 10% of each Convertible Note purchased by a Target and (ii) a non-accountable expense allowance in the aggregate amount of $127,500, such amount equal to 3% of each Convertible Note purchased by a Target.
In connection with the 2017 Private Placement, we paid the Placement Agent and selected dealers an aggregate cash fee of $1,917,823, inclusive of a non-accountable expense allowance equal to $506,499, and we incurred approximately $150,000 of other expenses related to the financing. In addition, as part of its compensation for acting as placement agent for the 2017 Private Placement, (i) we issued royalty payment rights certificates (the “Placement Agent Royalty Payment Rights Certificates”) to the Placement Agent, and its designees, to receive, in the aggregate, 10% of the amount of payments paid to the holders of the Series A Convertible Preferred Stock (see “Description of Securities – Placement Agent Royalty Payment Rights”). and (ii) we issued warrants (the “Placement Agent Warrants”) to the Placement Agent, and its designees, to purchase 403,632 shares of our common stock with an exercise price of $5.00 per share. Such warrants contain a “cashless exercise” feature and are exercisable at any time prior to five years from the date of grant.
Competition
We do not believe that there are currently any direct competitors in the market, nor any known competing technology under development, using similar technology to our technology. Currently the major colonoscope manufacturers (i.e. Olympus, Pentax, Fuji) sell an irrigation pump that can pump fluid through the working channel of a colonoscope. The only intra-procedural device in the market, Cantel Medical’s Jet Prep, and another product in development similar to Cantel Medical’s Jet Prep, Medjet Ltd.’s MedJet, go through the working channel of a scope and are used mostly for spot cleaning a small amount of debris and do not have the capability to fully clean the colon of large amounts of fecal matter. The Jet Prep and MedJet products also require the physician to remove it from the working channel during the procedure if they need to remove polyps or take a biopsy, impacting the workflow of the procedure. The competitive products mentioned are not currently reimbursed by private or government payers. There are over ten different preparation regimes used prior to colonoscopy today. Some are prescription medications and others are over the counter. Typically, the over the counter regimes are not indicated for colonoscopy prep but for issues of motility, such as constipation, but are still widely prescribed by physicians for colonoscopy prep. Depending on the insurance a patient has, the prescription prep may be covered in part but many of them require the patient to pay out of pocket.
The medical device and pharmaceutical industries are intensely competitive and subject to rapid and significant technological change. We have indirect competitors in a number of jurisdictions, many of which have substantially greater name recognition, commercial infrastructures and financial, technical and personnel resources than we have. Currently, the colonoscopy market is dominated by Olympus, who controls a majority of the market, with Pentax and FujiFilm taking most of the rest of the US colonoscope market. Boston Scientific, Medtronic US Endoscopy, Medivators and other smaller players sell ancillary devices and accessories into the marketplace as well. These established competitors may invest heavily to quickly discover and develop novel devices that could make our Pure-Vu system obsolete or uneconomical. While colonoscopy remains the gold standard for CRC screening, there are capsule endoscopy systems such as the PillCamTM from Medronic and the Endocapsule 10 from Olympus, These systems, however, require at least the same level of prep as conventional colonoscopies, cannot remove polyps, and therefore, cannot be used to obtain biopsies to detect cancer, so may be less useful for colonoscopy as compared to visualization of the small bowel. Another visualization technique is the virtual colonoscopy, where a radiologist uses a CT scan to obtain 2D and 3D images of the colon. Virtual colonoscopies may require the same level of prep as conventional colonoscopies and if a polyp or abnormality is detected, the patient may still need to undergo a colonoscopy. Other screening tests for colon cancer specifically include fecal occult blood tests and DNA stool tests such as the Cologuard test from Exact Sciences. However, Cologuard is not a replacement for diagnostic colonoscopies or surveillance colonoscopies in high risk individuals and has a lower specificity than standard colonoscopies. While none of these testing alternatives may ever fully replace the colonoscopy, over time, they may take market share away from conventional colonoscopies for specific purposes and may lower the potential market opportunity for us.
| 47 |
Any new product that competes with an approved product may need to demonstrate compelling advantages in efficacy, cost, convenience, tolerability and safety to be commercially successful. Other competitive factors, including new competitive entrants, could force us to lower prices or could result in reduced sales. In addition, new products developed by others could emerge as competitors to the Pure-Vu system. If we are not able to compete effectively against our current and future competitors, our business will not grow and our financial condition and operations will suffer.
Research and Development
We incurred expenses of approximately $1.7 million and $1.8 million, respectively, during the six months ended June 30, 2017 and 2016 for research and development activities. We incurred expenses of approximately $3.1 million and $3.2 million, respectively, in 2016 and 2015 for research and development activities. These expenses include cash and non-cash expenses relating to the development of our development and clinical programs for the Pure-Vu system. We have research and development capabilities in electrical and mechanical engineering with laboratories in our facility in Israel for development and prototyping, and electronics design and testing. We also use consultants and third party design houses to complement our internal capabilities.
We have received grants from the Government of the State of Israel through the IIA (formerly known as the OCS) for the financing of a portion of our research and development expenditures pursuant to the Research Law and related regulations. As of September 30, 2017, we had received funding from the IIA in the aggregate amount of $1.33 million and had a contingent obligation to the IIA in the amount of approximately $1.41 million, which is generally repaid in the form of royalties ranging from 3% to 3.5% of revenues on sales of products and services based on technology developed using IIA grants, up to an aggregate of 100% (which may be increased under certain circumstances) of the U.S. dollar-linked value of the grant, plus interest at the rate of 12-month LIBOR. As of September 30, 2017, we paid a minimal amount to the IIA. We may apply for additional IIA grants in the future. However, as the funds available for the IIA grants out of the annual budget of the State of Israel have been reduced in the past and may be further reduced in the future, we cannot predict whether we will be entitled to any future grants, or the amounts of any such grants. The terms of the Israeli government participation also require that products developed with IIA grants be manufactured in Israel and that the technology developed thereunder may not be transferred outside of Israel (even following the full repayment of the IIA grants), unless prior approval is received from the IIA, which approval may not be granted. Even if such approval is granted, the transfer outside of Israel of manufacturing which is connected with IIA-funded know-how may result in increased royalty payments (up to three times the aggregate amount of the IIA grants plus interest thereon), as well as in a higher royalty repayment rate. In addition, the transfer outside of Israel of IIA-funded know-how may trigger additional payments to the IIA (up to six times the aggregate amount of the IIA grants plus interest thereon). For additional information, see “Business - Manufacturing and Supply” below.
Manufacturing and Supply
We currently have internal capabilities for small scale production in our facility in Israel. We have ISO 13485 certification for our quality system using DEKRA as our Notified Body in Europe. The internal capability will support the initial limited pilot launch of the product in the U.S. as we establish higher volume capabilities with external manufacturing partners. We are in the process of finalizing supply agreements with two different contract manufacturers, one for the workstation and the other for the disposable portion of the Pure-Vu system. The manufacturing suppliers we are negotiating with have extensive experience in medical devices and dealing with regulatory bodies. The suppliers we are targeting have ISO 13485 approved quality systems. We anticipate they will be producing Pure-Vu systems by the end of 2017. We have an agreement in place with a third party logistics provider in the US who is ISO 13485 certified and specializes in medical devices and equipment. They will provide warehousing, shipping and back office support to meet our commercial needs.
| 48 |
We have received grants from the Government of the State of Israel through the IIA (formerly known as the OCS) for the financing of a portion of our research and development expenditures, the terms of which require that products developed with IIA grants be manufactured in Israel and that the technology developed thereunder may not be transferred outside of Israel (including by way of certain licenses), unless prior approval is received from the IIA, which we intend to apply for but may not receive (and any such approval may be subject to increased royalty repayment rates and increased royalties). Even following the full repayment of any IIA grants, we must nevertheless continue to comply with the requirements of the Research Law. The foregoing restrictions may impair our ability to outsource or transfer development or manufacturing activities with respect to any product or technology outside of Israel. For additional information, see “Risk Factors - Risks Related to Our Operations in Israel.”
A significant amendment to the Research Law entered into effect on January 1, 2016, under which the IIA, a statutory government corporation, was established and replaced the OCS. Under such amendment, the IIA is authorized to establish rules concerning the ownership and exploitation of IIA/OCS-funded know-how (including with respect to restrictions on transfer of manufacturing activities and IIA-funded know-how outside of Israel), which may differ from the restrictive laws, regulations and guidelines as currently in effect (and which shall remain in effect until such rules have been established by the IIA). In May 2017, the IIA issued new rules applicable to Israeli companies that receive grants from the IIA or its predecessor, the OCS, which went into effect on July 1, 2017. As of the date hereof, we cannot predict the impact these new rules will have on us or how they will be implemented by the IIA. Furthermore, it is anticipated that additional rules will be published by the IIA and we cannot predict or estimate the changes (if any) that may be made to this legislation (including with respect to the acquisition of an IIA-funded entity or the transfer of manufacturing or ownership of IIA-funded technology).
We have established relationships with research facilities, contract manufacturing organizations, or CMO’s, and our collaborators to manufacture and supply our product for commercialization. The Pure-Vu system workstations and disposables are currently manufactured at our facility in Israel. We are planning to transfer the manufacturing of the Pure-Vu system workstations to Sanmina Corporation’s manufacturing facilities in Israel and the Pure-Vu system disposables to Polyzen’s manufacturing facilities in North Carolina, US. We anticipate transitioning the manufacturing activities during 2017 as we establish and scale up our manufacturing capabilities with these CMO’s.
U.S. Market Entry Strategy
We have initiated a limited pilot launch in the US market. Initial evaluation cases have been performed at six centers showing cleansing capabilities similar to our clinical trial experience. This pilot phase is expected to run through 2018 with the primary objectives of expanding our clinical evidence, developing a practice integration model and creating key reference centers in both the AEC and hospital in-patient settings. We intend to work with the initial accounts to perform onsite analysis to optimize the prep for various populations including screening, surveillance, diagnostic and in-patients. We are working with a third party logistics provider specializing in medical devices to provide front and back office support to successfully fulfill customer orders. Additionally our commercial organization is putting in place the infrastructure to track account progress and help provide accurate forecasting for operations. We anticipate the sales cycle to be in the range of six to twelve months. During our limited pilot market entry, we will refine our commercialization strategy and tactics prior to our full market launch which is expected in 2019. Our full market launch will focus on launching our second generation Pure-Vu platform (lower cost of goods, added features, and additional size for “slim” scopes), growing the top line revenues, scaling the commercial organization and expanding our clinical indications for use. We expect to develop strategic relationships to pursue OUS marketing opportunities and to initiate sales in the EU in 2019 and Japan, China and other Asian markets in 2020.
Employees
As of September 30, 2017 we had 36 full time employees. All of our employees are engaged in administration, finance, clinical, R&D, engineering, regulatory and sales and marketing functions. We believe our relations with our employees are good. We anticipate that the number of employees will grow as we scale our commercial capabilities. In addition, we utilize and will continue to utilize consultants, clinical research organizations and third parties to perform our pre-clinical studies, clinical studies, manufacturing and regulatory functions.
| 49 |
Under Israeli law, we and our employees in Israel are subject to Israeli protective labor provisions, including the length of the workday, minimum wages for employees, annual leave, sick pay, determination of severance pay and advance notice of termination of employment, as well as procedures for hiring and dismissing employees and equal opportunity and anti-discrimination laws. While none of our employees in Israel is party to any collective bargaining agreements, orders issued by the Israeli Ministry of Economy and Industry may make certain industry-wide collective bargaining agreements applicable to us. These agreements affect matters such as the length of the workday and week, recuperation pay, travel expenses and pension rights. We have never experienced labor-related work stoppages and believe that our relationships with our employees are a significant part of our operations and that we maintain a good and positive relationship with our employees.
Israeli law generally requires the payment of severance compensation by employers upon the retirement, death or dismissal of an employee. We fund our ongoing Israeli severance obligations by making monthly payments to insurance policies. All of our current employees in Israel have agreed that upon termination of their employment, they will be entitled to receive only the amounts accrued in the insurance policies with respect to severance pay.
Furthermore, Israeli employees and employers are required to pay predetermined sums to the National Insurance Institute, which is similar to the U.S. Social Security Administration. These amounts also include payments for national health insurance.
Facilities
We currently rent 7,732 square feet of space in Tirat Carmel, Israel. This facility consists of office space, laboratories and a class eight cleanroom. We entered the lease on January 1, 2015, and the lease is for a period of five-years.
On April 13, 2017, we entered into a lease for a facility in Fort Lauderdale, Florida, which we intend to begin occupying in October 2017. The facility will be initially 4,554 square feet, which will increase to 6,390 square feet by the second year of the lease. The term will run for seven years and two months from the date we begin to occupancy the facility. This facility will be used for office space as well as laboratories for both quality assurance and product development.
Legal Matters
We are not currently subject to any material legal proceedings; however, we may from time to time become a party to various legal proceedings arising in the ordinary course of our business. Although the results of litigation and claims cannot be predicted with certainty, as of the date of this prospectus, we do not believe we are party to any claim or litigation the outcome of which, if determined adversely to us, would individually or in the aggregate be reasonably expected to have a material adverse effect on our business. Regardless of the outcome, litigation can have an adverse impact on us because of defense and settlement costs, diversion of management resources and other factors.
Regulatory Matters
Government Regulation
Our business is subject to extensive federal, state, local and foreign laws and regulations, including those relating to the protection of the environment, health and safety. Some of the pertinent laws have not been definitively interpreted by the regulatory authorities or the courts, and their provisions are open to a variety of subjective interpretations. In addition, these laws and their interpretations are subject to change, or new laws may be enacted.
Both federal and state governmental agencies continue to subject the healthcare industry to intense regulatory scrutiny, including heightened civil and criminal enforcement efforts. We believe that we have structured our business operations and relationships with our customers to comply with all applicable legal requirements. However, it is possible that governmental entities or other third parties could interpret these laws differently and assert otherwise. We discuss below the statutes and regulations that are most relevant to our business.
| 50 |
U.S. Food and Drug Administration regulation of medical devices.
The FDCA and FDA regulations establish a comprehensive system for the regulation of medical devices intended for human use. Our products include medical devices that are subject to these, as well as other federal, state, local and foreign, laws and regulations. The FDA is responsible for enforcing the laws and regulations governing medical devices in the United States.
The FDA classifies medical devices into one of three classes (Class I, Class II, or Class III) depending on their level of risk and the types of controls that are necessary to ensure device safety and effectiveness. The class assignment is a factor in determining the type of premarketing submission or application, if any, that will be required before marketing in the United States.
| ● | Class I devices present a low risk and are not life-sustaining or life-supporting. The majority of Class I devices are subject only to “general controls” (e.g., prohibition against adulteration and misbranding, registration and listing, good manufacturing practices, labeling, and adverse event reporting. General controls are baseline requirements that apply to all classes of medical devices.) | |
| ● | Class II devices present a moderate risk and are devices for which general controls alone are not sufficient to provide a reasonable assurance of safety and effectiveness. Devices in Class II are subject to both general controls and “special controls” (e.g., special labeling, compliance with industry standards, and post market surveillance. Unless exempted, Class II devices typically require FDA clearance before marketing, through the premarket notification (510(k)) process.) | |
| ● | Class III devices present the highest risk. These devices generally are life-sustaining, life-supporting, or for a use that is of substantial importance in preventing impairment of human health, or present a potential unreasonable risk of illness or injury. Class III devices are devices for which general controls, by themselves, are insufficient and for which there is insufficient information to establish special controls to provide a reasonable assurance of safety and effectiveness. Class III devices are subject to general controls and typically require FDA approval of a premarket approval (“PMA”) application before marketing. |
Unless it is exempt from premarket review requirements, a medical device must receive marketing authorization from the FDA prior to being commercially marketed, distributed or sold in the United States. The most common pathways for obtaining marketing authorization are 510(k) clearance and PMA.
510(k) pathway
The 510(k) review process compares a new device to a legally marketed device. Through the 510(k) process, the FDA determines whether a new medical device is “substantially equivalent” to a legally marketed device (i.e., predicate device) that is not subject to PMA requirements. “Substantial equivalence” means that the proposed device has the same intended use as the predicate device, and the same or similar technological characteristics, or if there are differences in technological characteristics, the differences do not raise different questions of safety and effectiveness as compared to the predicate, and the information submitted in the 510(k) demonstrates that the proposed device is as safe and effective as the predicate device.
To obtain 510(k) clearance, a company must submit a 510(k) application containing sufficient information and data to demonstrate that its proposed device is substantially equivalent to a legally marketed predicate device. These data generally include non-clinical performance testing (e.g., software validation, animal testing electrical safety testing), but may also include clinical data. Typically, it takes three to twelve months for the FDA to complete its review of a 510(k) submission; however, it can take significantly longer and clearance is never assured. During its review of a 510(k), the FDA may request additional information, including clinical data, which may significantly prolong the review process. After completing its review of a 510(k), the FDA may issue an order, in the form of a letter, that finds the device to be either (1) substantially equivalent and states that the device can be marketed in the United States, or (2) not substantially equivalent and states that device cannot be marketed in the United States. Depending upon the reasons for the not substantially equivalent finding, the device may need to be approved through the PMA pathway (discussed below) prior to commercialization.
| 51 |
After a device receives 510(k) clearance, any modification that could significantly affect the safety or effectiveness of the device, or that would constitute a major change in its intended use, including significant modifications to any of our products or procedures, requires submission and clearance of a new 510(k). The FDA relies on each manufacturer to make and document this determination initially, but the FDA can review any such decision and can disagree with a manufacturer’s determination. We may make minor product enhancements that we believe do not require new 510(k) clearances. If the FDA disagrees with our determination regarding whether a new 510(k) clearance was required for these modifications, we may need to cease marketing and/or recall the modified device. The FDA may also subject us to other enforcement actions, including, but not limited to, issuing a warning letter or untitled letter to us, seizing our products, imposing civil penalties, or initiating criminal prosecution.
Premarket approval pathway
Unlike the comparative standard of the 510(k) pathway, the PMA approval process requires an independent demonstration of the safety and effectiveness of a device. PMA is the most stringent type of device marketing application required by the FDA. PMA approval is based on a determination by the FDA that the PMA contains sufficient valid scientific evidence to ensure that the device is safe and effective for its intended use(s). A PMA application generally includes extensive information about the device including the results of clinical testing conducted on the device and a detailed description of the manufacturing process.
After a PMA application is accepted for review, the FDA begins an in-depth review of the submitted information. FDA regulations provide 180 days to review the PMA and make a determination; however, in reality, the review time is normally longer (e.g., 1-3 years). During this review period, the FDA may request additional information or clarification of information already provided. Also during the review period, an advisory panel of experts from outside the FDA may be convened to review and evaluate the data supporting the application and provide recommendations to the FDA as to whether the data provide a reasonable assurance that the device is safe and effective for its intended use. In addition, the FDA generally will conduct a preapproval inspection of the manufacturing facility to ensure compliance with QSR, which imposes comprehensive development, testing, control, documentation and other quality assurance requirements for the design and manufacturing of a medical device.
Based on its review, the FDA may (1) issue an order approving the PMA, (2) issue a letter stating the PMA is “approvable” (e.g., minor additional information is needed), (3) issue a letter stating the PMA is “not approvable,” or (4) issue an order denying PMA. A company may not market a device subject to PMA review until the FDA issues an order approving the PMA. As part of a PMA approval, the FDA may impose post-approval conditions intended to ensure the continued safety and effectiveness of the device including, among other things, restrictions on labeling, promotion, sale and distribution, and requiring the collection of additional clinical data. Failure to comply with the conditions of approval can result in materially adverse enforcement action, including withdrawal of the approval.
Most modifications to a PMA approved device, including changes to the design, labeling, or manufacturing process, require prior approval before being implemented. Prior approval is obtained through submission of a PMA supplement. The type of information required to support a PMA supplement and the FDA’s time for review of a PMA supplement vary depending on the nature of the modification.
Clinical trials
Clinical trials of medical devices in the United States are governed by the FDA’s Investigational Device Exemption (“IDE”) regulation. This regulation places significant responsibility on the sponsor of the clinical study including, but not limited to, choosing qualified investigators, monitoring the trial, submitting required reports, maintaining required records, and assuring investigators obtain informed consent, comply with the study protocol, control the disposition of the investigational device, submit required reports, etc.
| 52 |
Clinical trials of significant risk devices (e.g., implants, devices used in supporting or sustaining human life, devices of substantial importance in diagnosing, curing, mitigating or treating disease or otherwise preventing impairment of human health) require FDA and Institutional Review Board (“IRB”), approval prior to starting the trial. FDA approval is obtained through submission of an IDE application. Clinical trials of non-significant risk (“NSR”), devices (i.e. devices that do not meet the regulatory definition of a significant risk device) only require IRB approval before starting. The clinical trial sponsor is responsible for making the initial determination of whether a clinical study is significant risk or NSR; however, a reviewing IRB and/or FDA may review this decision and disagree with the determination.
An IDE application must be supported by appropriate data, such as performance data, animal and laboratory testing results, showing that it is safe to evaluate the device in humans and that the clinical study protocol is scientifically sound. There is no assurance that submission of an IDE will result in the ability to commence clinical trials. Additionally, after a trial begins, the FDA may place it on hold or terminate it if, among other reasons, it concludes that the clinical subjects are exposed to an unacceptable health risk.
As noted above, the FDA may require a company to collect clinical data on a device in the postmarket setting.
The collection of such data may be required as a condition of PMA approval. The FDA also has the authority to order, via a letter, a postmarket surveillance study for certain devices at any time after they have been cleared or approved.
Pervasive and continuing FDA regulation
After a device is placed on the market, regardless of its classification or premarket pathway, numerous additional FDA requirements generally apply. These include, but are not limited to:
| ● | Establishment registration and device listing requirements; | |
| ● | Quality System Regulation (“QSR”), which governs the methods used in, and the facilities and controls used for, the design, manufacture, packaging, labeling, storage, installation, and servicing of finished devices; | |
| ● | Labeling requirements, which mandate the inclusion of certain content in device labels and labeling, and generally require the label and package of medical devices to include a unique device identifier (“UDI”), and which also prohibit the promotion of products for uncleared or unapproved, i.e., “off-label,” uses; | |
| ● | Medical Device Reporting (“MDR”), regulation, which requires that manufacturers and importers report to the FDA if their device may have caused or contributed to a death or serious injury or malfunctioned in a way that would likely cause or contribute to a death or serious injury if it were to recur; and | |
| ● | Reports of Corrections and Removals regulation, which requires that manufacturers and importers report to the FDA recalls (i.e., corrections or removals) if undertaken to reduce a risk to health posed by the device or to remedy a violation of the FDCA that may present a risk to health; manufacturers and importers must keep records of recalls that they determine to be not reportable. |
| 53 |
The FDA enforces these requirements by inspection and market surveillance. Failure to comply with applicable regulatory requirements can result in enforcement action by the FDA, which may include, but is not limited to, the following sanctions:
| ● | Untitled letters or warning letters; | |
| ● | Fines, injunctions and civil penalties; | |
| ● | Recall or seizure of our products; | |
| ● | Operating restrictions, partial suspension or total shutdown of production; | |
| ● | Refusing our request for 510(k) clearance or premarket approval of new products; | |
| ● | Withdrawing 510(k) clearance or premarket approvals that are already granted; and | |
| ● | Criminal prosecution. |
We are subject to unannounced device inspections by the FDA, as well as other regulatory agencies overseeing the implementation of and compliance with applicable state public health regulations. These inspections may include our suppliers’ facilities.
International
International sales of medical devices are subject to foreign government regulations, which vary substantially from country to country. In order to market our products in other countries, we must obtain regulatory approvals and comply with extensive safety and quality regulations in other countries. The time required to obtain approval by a foreign country may be longer or shorter than that required for FDA clearance or approval, and the requirements may differ. The European Union/European Economic Area (the “EU/EEA”), requires a CE conformity mark in order to market medical devices. Many other countries, such as Australia, India, New Zealand, Pakistan and Sri Lanka, accept CE or FDA clearance or approval, although others, such as Brazil, Canada and Japan require separate regulatory filings.
In the EU and the EEA, devices are required to comply with the essential requirements of the EU Medical Devices Directive. Compliance with these requirements would entitle us to affix the CE conformity mark to our medical devices, without which they cannot be commercialized in the EU and EEA. To demonstrate compliance with the essential requirements and obtain the right to affix the CE conformity mark we must undergo a conformity assessment procedure, which varies according to the type of medical device and its classification. Except for low risk medical devices (Class I), where the manufacturer can issue a CE Declaration of Conformity based on a self-assessment of the conformity of its products with the essential requirements of the Medical Devices Directive, a conformity assessment procedure requires the intervention of a Notified Body, which is an organization accredited at the European Commission to conduct conformity assessments. The Notified Body would typically audit and examine the quality system for the manufacture, design and final inspection of our devices before issuing a certification demonstrating compliance with the essential requirements. Based on this certification we can draw up a CE Declaration of Conformity which allows us to affix the CE Mark to our products.
Further, the advertising and promotion of our products in the EU and the EEA is subject to the laws of individual EU and EEA Member States implementing the EU Medical Devices Directive, Directive 2006/114/EC concerning misleading and comparative advertising, and Directive 2005/29/EC on unfair commercial practices, as well as other EU and EEA Member State laws governing the advertising and promotion of medical devices. These laws may limit or restrict the advertising and promotion of our products to the general public and may impose limitations on our promotional activities with healthcare professionals.
Other Regulatory Matters
Manufacturing, sales, promotion and other activities following product approval are also subject to regulation by numerous regulatory authorities in addition to the FDA, including, in the United States, the Centers for Medicare & Medicaid Services (“CMS”), other divisions of the Department of Health and Human Services, the Drug Enforcement Administration, the Consumer Product Safety Commission, the Federal Trade Commission, the Occupational Safety & Health Administration, the Environmental Protection Agency, and state and local governments. Sales, marketing and scientific/educational programs must also comply with federal and state fraud and abuse laws. Pricing and rebate programs must comply with the Medicaid rebate requirements of the U.S. Omnibus Budget Reconciliation Act of 1990. If products are made available to authorized users of the Federal Supply Schedule of the General Services Administration, additional laws and requirements apply. Manufacturing, sales, promotion and other activities are also potentially subject to federal and state consumer protection and unfair completion laws.
| 54 |
The distribution of medical device products is subject to additional requirements and regulations, including extensive record-keeping, licensing, storage and security requirements intended to prevent the unauthorized sale of medical device products.
Third-Party Payer Coverage and Reimbursement
Our Pure-Vu system and the procedure to cleanse the colon in preparation for colonoscopy are not currently reimbursable through private or governmental third-party payors in any country. Significant uncertainty exists as to whether coverage and reimbursement of the Pure-Vu system will develop; but we do intend to seek reimbursement through private or governmental third-party payors in the future. In both the United States and foreign markets, our ability to commercialize the Pure-Vu system successfully, and to attract commercialization partners for the Pure-Vu system, depends in part on the availability of adequate financial coverage and reimbursement from third-party payers, including, in the United States, governmental payers such as the Medicare and Medicaid programs, managed care organizations, and private health insurers. Medicare is a federally funded program managed by the CMS, through local fiscal intermediaries and carriers that administer coverage and reimbursement for certain healthcare items and services furnished to the elderly and disabled. Medicaid is an insurance program for certain categories of patients whose income and assets fall below state defined levels and who are otherwise uninsured that is both federally and state funded and managed by each state. The federal government sets general guidelines for Medicaid and each state creates specific regulations that govern its individual program. Each payer has its own process and standards for determining whether it will cover and reimburse a procedure or particular product. Private payers often rely on the lead of the governmental payers in rendering coverage and reimbursement determinations. Therefore, achieving favorable CMS coverage and reimbursement is usually a significant gating issue for successful introduction of a new product. The competitive position of the Pure-Vu system will depend, in part, upon the extent of coverage and adequate reimbursement for such product and for the procedures in which such product is used. Prices at which we or our customers seek reimbursement for the Pure-Vu system can be subject to challenge, reduction or denial by the government and other payers.
In addition, in some foreign countries, the proposed pricing for a medical device must be approved before it may be lawfully marketed. The requirements governing drug pricing vary widely from country to country. For example, the European Union provides options for its member states to restrict the range of medicinal products for which their national health insurance systems provide reimbursement and to control the prices of medicinal products for human use. A member state may approve a specific price for the medicinal product or it may instead adopt a system of direct or indirect controls on the profitability of the company placing the medicinal product on the market. Historically, products launched in the European Union do not follow price structures of the United States and generally tend to be significantly lower.
Other Healthcare Laws and Compliance Requirements
In the United States, our activities are potentially subject to regulation by various federal, state and local authorities in addition to the FDA, including the CMS, other divisions of the United States Department of Health and Human Services (e.g., the Office of Inspector General), the United States Department of Justice and individual United States Attorney offices within the Department of Justice, and state and local governments. These regulations include:
| ● | the federal healthcare program anti-kickback law which prohibits, among other things, persons from soliciting, receiving or providing remuneration, directly or indirectly, to induce either the referral of an individual, for an item or service or the purchasing or ordering of a good or service, for which payment may be made under federal healthcare programs such as the Medicare and Medicaid programs; | |
| ● | federal false claims laws which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid, or other government reimbursement programs that are false or fraudulent. The government may assert that a claim including items or services resulting from a violation of the federal healthcare program anti-kickback law or related to off-label promotion constitutes a false or fraudulent claim for purposes of the federal false claims laws; |
| 55 |
| ● | the federal Health Insurance Portability and Accountability Act of 1996 which prohibits executing a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters and which also imposes certain requirements relating to the privacy, security and transmission of individually identifiable health information; | |
| ● | the federal transparency requirements under the Health Care Reform Law requires manufacturers of drugs, devices, biologics, and medical supplies to report to the Department of Health and Human Services information related to physician payments and other transfers of value and physician ownership and investment interests; | |
| ● | the Federal Physician Payments Sunshine Act within the Patient Protection and Affordable Care Act, and its implementing regulations, require that certain manufacturers of drugs, devices, biological and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program (with certain exceptions) to report information related to certain payments or other transfers of value made or distributed to physicians and teaching hospitals, or to entities or individuals at the request of, or designated on behalf of, the physicians and teaching hospitals and to report annually certain ownership and investment interests held by physicians and their immediate family members; and | |
| ● | state law equivalents of each of the above federal laws, such as anti-kickback and false claims laws, which may apply to items or services reimbursed by any third party payer, including commercial insurers, and state laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and often are not preempted by federal laws, thus complicating compliance efforts. |
In addition, we may be subject to data privacy and security regulation by both the federal government and the states in which we conduct our business. The Health Insurance Portability and Accountability Act, or HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, or HITECH, and its implementing regulations, imposes certain requirements relating to the privacy, security and transmission of individually identifiable health information. Among other things, HITECH makes HIPAA’s privacy and security standards directly applicable to “business associates”—independent contractors or agents of covered entities that receive or obtain protected health information in connection with providing a service on behalf of a covered entity. HITECH also created four new tiers of civil monetary penalties, amended HIPAA to make civil and criminal penalties directly applicable to business associates and possibly other persons, and gave state attorneys general new authority to file civil actions for damages or injunctions in federal courts to enforce the federal HIPAA laws and seek attorneys’ fees and costs associated with pursuing federal civil actions. In addition, state laws govern the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts.
Post-Marketing Regulations
Following approval of a new product, a company and the approved product are subject to continuing regulation by the FDA and other federal and state regulatory authorities, including, among other things, monitoring and recordkeeping activities, reporting to applicable regulatory authorities of adverse experiences with the product, providing the regulatory authorities with updated safety and efficacy information, product sampling and distribution requirements, and complying with promotion and advertising requirements, which include, among others, standards for direct-to-consumer advertising, restrictions on promoting for uses or in patient populations not described in the product’s approved labeling (known as “off-label use”), limitations on industry-sponsored scientific and educational activities, and requirements for promotional activities involving the internet. Although physicians may prescribe legally available products for off-label uses, manufacturers may not market or promote such off-label uses. Modifications or enhancements to the products or labeling or changes of site of manufacture are often subject to the approval of the FDA and other regulators, which may or may not be received or may result in a lengthy review process.
| 56 |
MANAGEMENT AND BOARD OF DIRECTORS
The following sets forth certain information with respect to our officers and directors.
| Name | Age | Position(s) | ||
| Mark Pomeranz | 56 | Chief Executive Officer and Director | ||
| Andrew Taylor | 46 | Chief Financial Officer | ||
| David Hochman | 42 | Chairman of the Board | ||
| Darren Sherman | 46 | Director | ||
| Gary Jacobs | 60 | Director | ||
| Samuel Nussbaum | 69 | Director | ||
| Shervin Korangy | 42 | Director |
Management
Mark Pomeranz, Chief Executive Officer, Director
Mr. Pomeranz has been Chief Executive Officer of Opco since 2014 and has served as our CEO since the Share Exchange Transaction. Prior to joining Opco, from 2007 to 2014, Mr. Pomeranz was the founding CEO of Svelte Medical Systems, a start-up company that is currently commercializing a unique drug eluting stent platform in the EU. From 1998 to 2007, Mr. Pomeranz served as Vice President at Cordis, a Johnson & Johnson Company, where his responsibilities included developing new technologies, exploring new market opportunities and leading major restructuring efforts to create cross-functional global commercialization teams. Prior to that, Mr. Pomeranz held a number of senior leadership roles, including positions at Cardiac Pathways Corporations from 1991 to 1998, and Cardiovascular Imaging Systems from 1989 to 1991, both of which were acquired by Boston Scientific Corporation. Mr. Pomeranz currently has 39 issued US patents and 15 pending in the areas of angioplasty catheter design, intravascular ultrasound devices, cooled tip and saline mediated RF ablation, high density cardiac mapping, embolic devices and others. He has authored multiple publications in the areas of electrophysiology, neurovascular treatments and percutaneous vascular interventions. Mr. Pomeranz earned a M.Sc. in biomedical engineering from the University of Miami. Mr. Pomeranz was selected as a director due to his history as a director of Opco and his business and leadership experience in the medical technology sector; his broad scientific background is also seen as an asset to us.
Andrew Taylor, Chief Financial Officer
Mr. Taylor has served as our Chief Financial Officer since August 2017. Prior to joining us, Mr. Taylor served as the CFO and President of Angel Medical Systems from 2007 until 2017. Angel Medical Systems is a medical device company that develops and manufactures continuous intra-cardiac ischemia monitoring and alerting systems. While at Angel Medical Systems, Mr. Taylor supervised the operations of more than fifty (50) employees in the United States and Brazil, while also overseeing the financial planning and analysis activities, capital raise efforts, and implementation of capital and operating budgets. From 2005 to 2007, Mr. Taylor was a Practice Leader for AC Lordi Consulting, where he oversaw staff providing CFO and Controller consulting services. Prior to that, Mr. Taylor was the CFO of Safe3w, Inc. from 2001 to 2005 until its acquisition by iPass, Inc. (NASDAQ: IPAS), where he led all accounting and finance functions as well as the fundraising efforts, and negotiated the sale of the company. From 1999 to 2001, Mr. Taylor served as the Vice President of Finance and Administration of Abridge, Inc., where he developed and managed processes for budgeting, forecasting and cash management. Prior to that, Mr. Taylor was a Senior Finance Associate and Business Analyst at Delta Air Lines (NYSE: DAL), from 1997 to 1999. Mr. Taylor is a CFA Program Level II Candidate and earned a B.A. in Political Science and Economics at McGill University and his MBA in Finance at Northeastern University.
Directors
Mark Pomeranz, Chief Executive Officer and Director
See description under Management.
| 57 |
David P. Hochman, Chairman of the Board
Mr. Hochman has been Chairman of the Board of Opco since 2011 and has served on our board of directors as Chairman since the Share Exchange Transaction. Since June 2006, Mr. Hochman has been Managing Partner of Orchestra Medical Ventures, LLC, an investment firm that employs a strategy to create, build and invest in medical technology companies intended to generate substantial clinical value and investor returns. He is also President of Accelerated Technologies, Inc., a medical device accelerator company managed by Orchestra. He has twenty years of venture capital and investment banking experience. He is Chairman of Caliber Therapeutics and a director of BackBeat Medical, Inc. (where he is also President), and FreeHold Surgical, Inc., all of which are Orchestra portfolio companies. Mr. Hochman currently serves as a board member of Corbus Pharmaceuticals Holdings, Inc. (NASDAQ: CRBP), a clinical stage biopharmaceutical company focused on the development and commercialization of novel therapeutics to treat rare, life-threating inflammatory-fibrotic diseases with clear unmet medical needs. He serves as a director of Adgero Biopharmaceuticals Holdings, Inc. and is the Vice Chairman and a Director of Naked Brand Group Inc. (Nasdaq: NAKD). Prior to joining Orchestra, Mr. Hochman was Chief Executive Officer of Spencer Trask Edison Partners, LLC, an investment partnership focused on early stage healthcare companies. He was also Managing Director of Spencer Trask Ventures, Inc. during which time he led financing transactions for over twenty early-stage companies. Mr. Hochman was a board advisor of Health Dialog Services Corporation, a leader in collaborative healthcare management that was acquired in 2008 by the British United Provident Association for $750 million. From 2005 to 2007, he was a co-founder and board member of PROLOR Biotech, Inc., a biopharmaceutical company developing longer-lasting versions of approved therapeutic proteins, which was purchased by Opko Health (NYSE: OPK) in 2013 for over $600 million. He currently serves on the board of two non-profit organizations: the Citizens Committee for New York City and the Mollie Parnis Livingston Foundation. He has a B.A. degree with honors from the University of Michigan. Mr. Hochman was selected as a director due to his history as a director of Opco, his leadership experience at other public companies, including medical technology companies, his financial experience and his expertise in governance matters.
Darren Sherman, Director
Mr. Sherman has been a director of Opco since 2015 and has served on our board of directors since the Share Exchange Transaction. Since 2009, Mr. Sherman has been a Managing Partner of Orchestra Medical Ventures, LLC, an investment firm that employs a strategy to create, build and invest in medical technology companies intended to generate substantial clinical value and investor returns. He has also served as Chief Technology Officer of Accelerated Technologies, Inc., a medical device accelerator company managed by Orchestra, since 2008. Mr. Sherman has over 20 years of management and entrepreneurial experience in the medical technology industry spanning interventional cardiology, cardiac electrophysiology, sudden cardiac death, stroke, surgery, GI, and neurovascular therapies. He is the CEO and a Director of Caliber Therapeutics, Inc., CEO and a Director of FreeHold Surgical, Inc., and a Director of BackBeat Medical, Inc., all of which are Orchestra portfolio companies. Prior to joining Orchestra, from February 2002 until March 2008, Mr. Sherman held positions in executive management for Cordis Neurovascular (CNV), a Johnson & Johnson company, including Executive Director R&D and Director of Strategic Marketing for stroke products. He had responsibility for all neurovascular R&D and global strategic marketing responsibilities for the stroke franchise, including budgets, a portfolio of products and strategic planning. Mr. Sherman made contributions to the design and commercialization of a series of products including the Enterprise Vascular Reconstruction Device and the Orbit Embolic Coil. From January 1997 until February 2002, Mr. Sherman was involved in the formation and development of Revivant Corp (acquired by Zoll Medical Corporation) while working at Fogarty Engineering. At Revivant, he managed the design, development, and testing of the AutoPulse device from concept through market introduction. From January 1995 until January 1997, Mr. Sherman held positions in research and development for Cardiac Pathways Corp., prior to its acquisition by Boston Scientific, and Baxter Healthcare. Mr. Sherman has authored more than sixty-five U.S. patents and has over eighty additional published applications. He earned a BS degree in Bioengineering from the University of California, San Diego. Mr. Sherman was selected as a director due to his history as a director of Opco and his leadership experience at other companies, including medical technology companies.
| 58 |
Gary Jacobs, Director
Mr. Jacobs has been a director of Opco since 2011 and has served on our board of directors since the Share Exchange Transaction. Mr. Jacobs is the Founder and Managing Director at Jacobs Investment Company, LLC, and served as Chief Executive Officer of DermTech, Inc. (DermTech International). He owns and operates a professional minor league baseball team, the Lake Elsinore Storm, affiliated with the San Diego Padres. Mr. Jacobs served as a software engineer and senior education specialist of QUALCOMM, Inc. and as a software programmer at Linkabit Incorporated. He has multi-million dollar investments in several other venture capital funds. He has been the Chairman of DermTech International since 2006 and serves as the Chairman of GEO2 Technologies Inc., Ora Bio Ltd. and High Tech High, the National High School Reform movement. He serves as Vice Chairman of the Jewish Community Center Association Continental Board. He has been a Director of Fallbrook Technologies, Inc. since March 31, 2004. He serves as a Director of New Generation Technology, Next Generation Technologies, Bio2 Technologies, Inc., Nutrinia Ltd., San Diego Symphony, Lawrence Family JCC and UCSD Board of Overseers. He serves as a Director of NGT3 and ParaSonic Ltd. He serves as a Director of Padres L.P., Viryd Technologies, Inc. and DermTech International. In addition, Mr. Jacobs serves as Chair of the Dean’s Advisory Council for Social Sciences at the University of California, San Diego. His other philanthropic work included as the President of the United Jewish Federation of San Diego County. Mr. Jacobs received his B.A. in Management Science from the University of California, San Diego in 1979. Mr. Jacobs was selected as a director due to his history as a director of Opco, his extensive experience serving on the board of directors of other companies, including medical technology companies, and his financial experience.
Samuel R. Nussbaum, M.D., Director
Dr. Nussbaum has served on our board of directors since the Share Exchange Transaction. During 2016 Dr. Nussbaum began serving as a Strategic Consultant for EBG Advisors, the consulting arm for Epstein Becker and Green, where he advises life science companies, health care systems and provider organizations. Dr. Nussbaum also serves as a Senior Advisor to Sandbox Industries Ventures, a venture capital firm, and Ontario Teachers Pension Fund. He is a member of the Scientific Advisory Board of Medidata (NASDAQ: MDSO), a publicly traded clinical technology company serving life sciences clients, and the Healthcare Advisory Board of KPMG. From 2000 until 2016, Dr. Nussbaum served as Executive Vice President, Clinical Health Policy, and Chief Medical Officer of Anthem, Inc. (NYSE: ANTM), where he was responsible for annual health care expenditures through business units focused on care management, health improvement, and provider network contracting. Prior to joining Anthem, Dr. Nussbaum served as executive vice president, Medical Affairs and System Integration of BJC Health Care, where he led integrated clinical services and community health, served as President of its medical group and chairman of its commercial (HealthPartners of the Midwest) and Medicaid (CarePartners) health plans. He currently serves on the Board of Directors of New England Healthcare Institute (NEHI), BioCrossroads (an Indiana-based public-private collaboration that advances and invests in the life sciences), and America’s Agenda. Dr. Nussbaum has also served on the Board of Directors of CareNex Health Services, National Quality Forum, America’s Health Insurance Plans (AHIP), Regenstrief Institute, National Committee for Quality Health Care, the OASIS Institute, VHA Foundation Board, Barnes-Jewish West County Hospital Board, Barnes-Jewish St. Peters Hospital Board, United Way of Greater St. Louis, and the Battelle Advisory Board. Dr. Nussbaum earned his BA from New York University and his MD from Mount Sinai School of Medicine. He trained in internal medicine at Stanford University and Massachusetts General Hospital and in endocrinology at Harvard Medical School and Massachusetts General Hospital. Dr. Nussbaum was selected as a director because of his medical and business experience in the healthcare and life sciences industries.
Shervin J. Korangy, Director
Mr. Korangy has served on our board of directors since March 2017. Mr. Korangy also serves as the Chief Financial Officer and Chief Strategy Officer of Beaver-Visitec International, a leading global developer, manufacturer and marketer of specialized surgical devices for the ophthalmic marketplace. From 2012 to 2017, Mr. Korangy served as a General Manager for the Alcon division of Novartis Group AG (NYSE: NVS), a global healthcare company, where he works with medical device, pharmaceutical and consumer health product segments. While part of Novartis Group AG, from 2010 to 2012, Mr. Korangy was the Global Head of Corporate Finance, where he was responsible for M&A strategy and supervised the acquisition of Alcon. Mr. Korangy is a current member of the Board of Directors of Pelican Rouge, a coffee branding and vending business and Sight Sciences LLC, a medical start-up business. From 1996 to 2010, Mr. Korangy worked in the Private Equity and Restructuring Advisory divisions of the Blackstone Group (NYSE: BX), where he served as a Managing Director. Mr. Korangy is a former member of the Board of Directors of Ultra Music, an electronic and dance music record label, Graham Packaging, a manufacturer and distributer of custom plastic containers for consumer product companies, Pinnacle Foods (NYSE: PF), a consumer packaged foods manufacturer and distributer and Bayview Financial, an asset manager and loan servicer. Mr. Korangy received his B.S. degree in economics at the Wharton School of the University of Pennsylvania, where he graduated magna cum laude. Mr. Korangy was selected as a director due to his management experience with medical device, pharmaceutical and consumer health products, and his financial and accounting experience.
| 59 |
Committees of the Board of Directors
Our board of directors has established an Audit Committee, a Compensation Committee, and a Nominating and Corporate Governance Committee. Our board of directors may establish other committees to facilitate the management of our business. The composition and functions of each committee are described below. Members serve on these committees until their resignation or until otherwise determined by our board of directors. Each of these committees operate under a charter that has been approved by our board of directors, which will be available on our website.
Audit Committee. Our Audit Committee consists of Mr. Korangy , Mr. Jacobs and Mr. Sherman, with Mr. Korangy serving as the Chairman of the Audit Committee. Our board of directors has determined that the three directors currently serving on our Audit Committee are independent within the meaning of the NASDAQ Marketplace Rules and Rule 10A-3 under the Exchange Act. In addition, our board of directors has determined that Mr. Korangy qualifies as an audit committee financial expert within the meaning of SEC regulations and The NASDAQ Marketplace Rules.
The Audit Committee oversees and monitors our financial reporting process and internal control system, reviews and evaluates the audit performed by our registered independent public accountants and reports to the board of directors any substantive issues found during the audit. The Audit Committee is directly responsible for the appointment, compensation and oversight of the work of our registered independent public accountants. The Audit Committee reviews and approves all transactions with affiliated parties.
Compensation Committee. Our Compensation Committee consists of Mr. Hochman, Mr. Jacobs and Mr. Nussbaum, with Mr. Hochman serving as the Chairman of the Compensation Committee. Our board of directors has determined that the three directors currently servicing on our Compensation Committee are independent under the listing standards, are “non-employee directors” as defined in rule 16b-3 promulgated under the Exchange Act and are “outside directors” as that term is defined in Section 162(m) of the Internal Revenue Code of 1986, as amended.
The Compensation Committee provides advice and makes recommendations to the board of directors in the areas of employee salaries, benefit programs and director compensation. The Compensation Committee also reviews and approves corporate goals and objectives relevant to the compensation of our President, Chief Executive Officer, and other officers and makes recommendations in that regard to the board of directors as a whole.
Nominating and Corporate Governance Committee. Our Nominating and Corporate Governance Committee consists of Mr. Sherman, Mr. Jacobs and Mr. Nussbaum, with Mr. Sherman serving as the Chairman of the Nominating and Corporate Governance Committee. The Nominating and Corporate Governance Committee nominates individuals to be elected to the board of directors by our stockholders. The Nominating and Corporate Governance Committee considers recommendations from stockholders if submitted in a timely manner in accordance with the procedures set forth in our bylaws and will apply the same criteria to all persons being considered. All members of the Nominating and Corporate Governance Committee are independent directors as defined under the NASDAQ listing standards.
| 60 |
Director Independence
Our board of directors undertook a review of its composition, the composition of its committees and the independence of each director. Based upon information requested from and provided by each director concerning his or her background, employment and affiliations, including family relationships, our board of directors has determined that Mr. Hochman, Mr. Sherman, Mr. Jacobs, Dr. Nussbaum, and Mr. Korangy do not have a relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director and that each of these directors is “independent” as that term is defined under the Rules of the NASDAQ Stock Market and the SEC.
Medical Advisory Board
We believe in seeking and attracting scientific and clinical leaders to provide counsel and support our growth. Our medical advisory board includes the following individuals, selected for their expertise in fields relating to gastroenterology and endoscopy, and we expect to add additional members in the future. Members of the medical advisory board meet with members of management and the board of directors to advise on scientific, product development and marketing matters. Each member of the medical advisory board is compensated on an hourly basis for services performed at our request, and is expected to attend at least one advisor board meeting per year, and to be available for at least two hours a month to provide feedback on clinical trial designs and new product designs.
Steven A. Edmundowicz, MD, FASGE
Dr. Steven Edmundowicz is the Medical Director of the Digestive Health Center at the University of Colorado Hospital and Professor of Medicine at the University of Colorado School of Medicine in Aurora, Colorado. He received his medical degree from Jefferson Medical College in Philadelphia, Pennsylvania, and completed residency training in internal medicine at Washington University School of Medicine, where he also completed a fellowship in gastroenterology. Dr. Edmundowicz is active in clinical practice, clinical investigation, teaching, and administration. He is a consultant and advisory board member for a number of medical device companies and has participated in a number of clinical trials in endoscopy, ERCP, and endoscopic ultrasound. Most recently, he has been involved with innovative endoscopic devices for use in the management of gastroesophageal reflux disease and morbid obesity. His clinical research involves the study and application of new technologies in endoscopy. In addition to his other responsibilities, Dr. Edmundowicz is the associate editor for ASGE News, and he was past senior associate editor of the journal Gastrointestinal Endoscopy. He maintains membership in the American Gastroenterological Association and American College of Gastroenterology and is a member of the ASGE Executive Committee and is the current ASGE treasurer.
Professor Ian M. Gralnek, MD, MSHS, FASGE
Professor Gralnek is Associate Professor of Medicine at the Rappaport Faculty of Medicine, Technion-Israel Institute of Technology and Chief, Institute of Gastroenterology and Hepatology at Ha’Emek Medical Center, Afula, Israel. He served his internship and residency in internal medicine at Hennepin County Medical Center in Minneapolis where he also served as Chief Resident. Professor Gralnek completed his fellowship in gastroenterology at UCLA Center for the Health Sciences. After receiving his Masters degree in Health Services from the UCLA School of Public Health, he completed a fellowship in health services research through UCLA, RAND & the West Los Angeles VA Medical Center. He has published more than 200 original papers, reviews, case reports, editorials, book chapters, and scientific abstracts. Professor Gralnek served as a counselor on the governing board of the American Society for Gastrointestinal Endoscopy (ASGE), Chairman of the International Committee for the ASGE, and as the Chairman of the ASGE Research Committee. He is a member of the European Society for Gastrointestinal Endoscopy (ESGE) Research and Education Committees and currently serves on the governing board of the ESGE. Professor Gralnek serves on the editorial boards of the American Journal of Gastroenterology, Gastroenterology and Hepatology Research, Archives of Gastroenterohepatology, and Current Treatment Options in Gastroenterology. He is also a Fellow of the American Society for Gastrointestinal Endoscopy.
| 61 |
Brian Jacobson, MD, MPH, AGAF, FASGE
Dr. Jacobson is the Medical Director of the Boston Accountable Care Organization (BACO), an ACO representing Boston Medical Center and several community health centers. He is an Associate Professor of Medicine at the Boston University School of Medicine and a practicing gastroenterologist at Boston Medical Center. Dr. Jacobson received his undergraduate degree from Amherst College, his medical degree from Albert Einstein College of Medicine, and his Masters Degree in Public Health from Harvard University School of Public Health. He completed both his residency in internal medicine and his fellowship in gastroenterology at Brigham and Women’s Hospital. He later served as Chief Medical Resident at Brigham and Women’s followed by a fellowship in advanced interventional endoscopy at the Brigham and Women’s and Massachusetts General Hospitals. Dr. Jacobson performs advanced endoscopic procedures and has published more than 100 scientific articles, including original research appearing in the New England Journal of Medicine, Gastroenterology and Gut. He participates in the training of fellows, residents, and medical students at Boston Medical Center and Boston University School of Medicine and is a Councilor on the Governing Board of the American Society for Gastrointestinal Endoscopy.
David Lieberman, MD, FACG
Dr. David Lieberman is Professor of Medicine and Chief of the Division of Gastroenterology and Hepatology at Oregon Health and Science University (OHSU) in Portland, Oregon and the Portland VA Medical Center. Dr. Lieberman is internationally recognized as an expert on colon cancer screening, with major research publications in New England Journal of Medicine, JAMA, Annals of Internal Medicine and Gastroenterology. Dr. Lieberman was the Chairman of the Multi-Society Task Force on Colorectal Cancer (2006-2012), and authored colon cancer screening guidelines in 2008 and polyp surveillance guideline in 2012 as well as colonoscopy quality indicators in 2007. He is the Director of the Clinical Outcomes Research Initiative (CORI), supported by NIH since 1999, which studies quality of endoscopy. Dr. Lieberman was Associate Editor of Gastroenterology (2011-2013) and was a member of the AGA Board (2012-2015) and currently serves as Vice President of AGA (2016-2017).
Ori Segol, MD
Dr. Ori Segol is a graduate of the Technion Institute of Haifa, Israel. He currently serves as Director of the Institute for the Digestive Tract, Carmel Medical Center, Haifa. Dr. Segol is highly experienced in performing advanced endoscopic procedures, including the removal of complex lesions in the digestive tract.
Professor Peter D. Siersema, MD, PhD, FASGE
Peter D. Siersema, MD, PhD is Professor of Endoscopic Gastrointestinal Oncology at the Radboud University Medical Center, Nijmegen, The Netherlands. His clinical interests include pre-malignant and malignant diseases of the gastrointestinal tract, especially esophageal cancer, hepato-biliary-pancreatic cancer and colorectal cancer. He is specialized in diagnostic and therapeutic endoscopy, i.e. endoscopic imaging, EMR/ESD, stent placement and ERCP. Dr. Siersema is President of the Dutch Society of Gastroenterology, Chair of the Committee for Revising the Dutch Gastroenterology Fellowship curriculum and Member of the Advisory Board of the Development and Innovation Committee of the Dutch Cancer Society. On an international level he is President of the European Society for Diseases of Esophagus (ESDE) and member of the Governing Board of the European Society for Gastrointestinal Endoscopy (ESGE). Dr. Siersema is Editor-in-Chief of the journal Endoscopy. He has authored more than 550 peer-reviewed papers and chapters in books, and has edited more than 20 books.
Gerald Bertiger, M.D.
Gerald Bertiger, MD, is the Managing Partner and President of Hillmont, GI, P.C. and Section Chief of Gastroenterology and Director of the Endoscopy Unit at Chestnut Hill Hospital. Dr. Bertiger is a clinical gastroenterologist who has been practicing in the northwest Philadelphia area for over 30 years. He completed his fellowship in gastroenterology at the Hospital of the University of Pennsylvania, and developed the first certified ambulatory endoscopy center in the state of Pennsylvania. Dr. Bertiger has developed his practice as a vertically integrated gastroenterology practice with lines of business in GI clinical practice, pathology, histology, anesthesia, ambulatory surgery and clinical research. He has consulted on medical affairs with companies in the pharmaceutical industry and served as a principal investigator for FDA monitored trials. His publications are in the areas of bowel preparations and basic motility research.
| 62 |
Compensation Committee Interlocks and Insider Participation
None of our executive officers serves, or in the past has served, as a member of the board of directors or compensation committee, or other committee serving an equivalent function, of any entity that has one or more executive officers who serve as members of our board of directors or our compensation committee. None of the members of our compensation committee is, or has ever been, an officer or employee of our company.
Code of Business Conduct and Ethics
We have adopted a written code of business conduct and ethics that applies to our employees, officers and directors. A current copy of the code will be posted on the Corporate Governance section of our website, which will be located at www.motusgi.com. We intend to disclose future amendments to certain provisions of our code of business conduct and ethics, or waivers of such provisions applicable to any principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions, and our directors, on our website identified above or in filings with the SEC.
Limitation of Directors Liability and Indemnification
The Delaware General Corporation Law authorizes corporations to limit or eliminate, subject to certain conditions, the personal liability of directors to corporations and their stockholders for monetary damages for breach of their fiduciary duties. Our certificate of incorporation limits the liability of our directors to the fullest extent permitted by Delaware law. In addition, we have entered into indemnification agreements with certain of our directors and officers whereby we have agreed to indemnify those directors and officers to the fullest extent permitted by law, including indemnification against expenses and liabilities incurred in legal proceedings to which the director or officer was, or is threatened to be made, a party by reason of the fact that such director or officer is or was a director, officer, employee or agent of the Company, provided that such director or officer acted in good faith and in a manner that the director or officer reasonably believed to be in, or not opposed to, the best interests of the Company.
We have director and officer liability insurance to cover liabilities our directors and officers may incur in connection with their services to us, including matters arising under the Securities Act. Our certificate of incorporation and bylaws also provide that we will indemnify our directors and officers who, by reason of the fact that he or she is or was one of our officers or directors of our Company, is involved in any action, suit or proceeding, whether civil, criminal, administrative or investigative related to their board role with the Company.
There is no pending litigation or proceeding involving any of our directors, officers, employees or agents in which indemnification will be required or permitted. We are not aware of any threatened litigation or proceeding that may result in a claim for such indemnification.
| 63 |
Summary Compensation Table
The following table presents information regarding the total compensation awarded to, earned by, or paid to our chief executive officer as of December 31, 2016 for services rendered in all capacities to us for the year ended December 31, 2016. This individual is our named executive officer for 2016.
| Name and Principal Position | Year | Salary ($) | Bonus ($) | Option Awards ($) | All other compensation ($) | Total ($) | ||||||||||||||||||
| Mark Pomeranz | 2016 | 350,000 | 70,000 | - | 36,211 | 456,211 | ||||||||||||||||||
Employment Agreements
In connection with the Share Exchange Transaction, we entered into an employment agreement with Mr. Pomeranz, which became effective on December 22, 2016 for a period of three years, which contains non-disclosure and invention assignment provisions. Under the terms of Mr. Pomeranz’s employment agreement, he holds the position of Chief Executive Officer, and is a member of the board of directors, and receives a base salary of $350,000 annually, subject to adjustments in the discretion of the board of directors; and he received a signing bonus of $70,000 upon the closing of the Share Exchange Transaction. In addition, Mr. Pomeranz is also eligible to receive an annual bonus, which is targeted at up to 25% of his base salary but which may be adjusted by our board of directors based on his individual performance and our performance as a whole. In connection with the final closing of the 2017 Private Placement, Mr. Pomeranz received a grant of options to purchase up to 511,113 shares of our common stock pursuant to our Equity Incentive Plan, of which fifty-three percent (53%) were fully vested when issued, forty percent (40%) will vest in a series of twelve (12) successive equal quarterly installments upon the completion of each successive calendar quarter of active service over the three (3) year period measured from the date of grant, as was determined by the Compensation Committee of the board of directors, and seven percent (7%) will not become fully vested until three years from the date of his employment agreement. In addition, pursuant to the terms of his employment agreement, Mr. Pomeranz is eligible to receive, from time to time, equity awards under our existing equity incentive plan, or any other equity incentive plan we may adopt in the future, and the terms and conditions of such awards, if any, will be determined by our Board of Directors or Compensation Committee, in their discretion. Mr. Pomeranz is also eligible to participate in any executive benefit plan or program we adopt.
On August 16, 2017, we entered into an employment agreement with Mr. Taylor, which became effective on August 16, 2017 (the “Commencement Date”) for a period of two years, which contains non-disclosure and invention assignment provisions. Under the terms of Mr. Taylor’s employment agreement, he holds the position of Chief Financial Officer and receives a base salary of $295,000 annually, subject to adjustments in the discretion of the board of directors; and he will be eligible to receive a signing bonus of $15,000 upon the date that is six (6) months following the Commencement Date. Mr. Taylor is also eligible to receive a relocation bonus of up to $35,000 if Mr. Taylor elects to relocate to Florida. In addition, Mr. Taylor is also eligible to receive a first year bonus payable following the one year anniversary of the Commencement Date, which is targeted at $30,000, and a second year bonus payable following the two year anniversary of the Commencement Date, which is targeted at $35,000, both of which may be adjusted by our board of directors based on his individual performance and our performance as a whole. In connection with his employment agreement, Mr. Taylor will receive a grant of options to purchase up to 240,000 shares of our common stock pursuant to our Equity Incentive Plan, which will vest in a series of twelve (12) successive equal quarterly installments upon the completion of each successive calendar quarter of active service over the three (3) year period measured from the date of grant, as determined by the Compensation Committee of the board of directors. In addition, pursuant to the terms of his employment agreement, Mr. Taylor is eligible to receive, from time to time, equity awards under our existing equity incentive plan, or any other equity incentive plan we may adopt in the future, and the terms and conditions of such awards, if any, will be determined by our Board of Directors or Compensation Committee, in their discretion. Mr. Taylor is also eligible to participate in any executive benefit plan or program we adopt.
| 64 |
The employment agreements with Israeli employees of Opco contain standard provisions for a company in our industry regarding non-competition, confidentiality of information and assignment of inventions. The enforceability of covenants not to compete in Israel is subject to limitations. For example, Israeli courts have recently required employers seeking to enforce non-compete undertakings of a former employee to demonstrate that the competitive activities of the former employee will harm one of a limited number of material interests of the employer which have been recognized by the courts, such as the secrecy of a company’s confidential commercial information or its intellectual property.
Outstanding Equity Awards at Fiscal Year-End
The following table summarizes, for each of the named executive officers, the number of shares of our common stock underlying outstanding stock options held as of December 31, 2016.
| Name | Number of Securities Underlying Unexercised Options (#) Exercisable | Equity Incentive Plan Awards: Number of Securities Underlying Un-exercised Unearned Options (#) | Option Exercise Price ($) | Option Expiration Date | ||||||
| Mark Pomeranz | 32,060 | (1) | 35,178 | (1) | 2.38 | March 26, 2024 | ||||
(1) Represents options to purchase shares of our common stock granted on March 26, 2014, under the Motus G.I. Medical Technologies LTD Employee Share Option Plan that were outstanding as of the Share Exchange Transaction, which were assumed by the 2016 Equity Incentive Plan (the “2016 Plan”) and continue in effect in accordance with their terms, on an adjusted basis to reflect the Share Exchange Transaction (see “Description of the 2016 Equity Incentive Plan - Administration” below). 48% of the option was vested as of December 31, 2016, with the remaining 52% of the option vesting upon the accomplishment of certain milestones.
Director Compensation
No compensation was paid to non-employee directors during 2016.
Non-Employee Director Compensation and Advisory Board Compensation
Our board of directors approved a director compensation policy for our directors, effective beginning July 1, 2017. This policy provides for the following cash compensation:
| ● | each director is entitled to receive a quarterly fee from us of $6,500; | |
| ● | the chairman of the Board will receive a quarterly fee from us of $9,000; | |
| ● | the chair of the Audit Committee will receive a quarterly fee from us of $2,500; and | |
| ● | each chair of any other board of director committee will receive a quarterly fee from us of $1,500. |
All fees under the director compensation policy will be paid on a quarterly basis in arrears and no per meeting fees will be paid. We will also reimburse non-employee directors for reasonable expenses incurred in connection with attending board of director and committee meetings.
Board Leadership Structure and Role in Risk Oversight
Risk is inherent with every business, and how well a business manages risk can ultimately determine its success. While the board of directors oversees risk management, our management is responsible for our day-to-day risk management process. Our board of directors has an active role, directly and through its committee structure, in the oversight of our risk management efforts.
| 65 |
Our board of directors satisfies this responsibility through full reports by each committee chair regarding the committee’s considerations and actions, as well as through regular reports directly from officers responsible for oversight of particular risks within our company. Our Audit Committee assists the board in performing its oversight responsibilities relating to our processes and policies with respect to identifying, monitoring, assessing, reporting on, managing and controlling our business and financial risk. The Audit Committee oversees, reviews, monitors and assesses (including through regular reports by, and discussions with, management), our processes and policies for risk identification, risk assessment, reporting on risk, risk management and risk control (including with respect to risks arising from our compensation policies and practices and in connection with the business and operations of its subsidiaries), and the steps that management has taken to identify, assess, monitor, report on, manage and control risks. The Audit Committee also discusses with management the balancing of risk versus reward for us and areas of specific risk identified by management and/or the Audit Committee.
Our board of directors believes that full and open communication between management and the board of directors is essential for effective risk management and oversight.
2016 Equity Incentive Plan
General
On December 14, 2016, our board of directors adopted the 2016 Plan having substantially the terms described herein.
The general purpose of the 2016 Plan is to provide a means whereby eligible employees, officers, non-employee directors and other individual service providers develop a sense of proprietorship and personal involvement in our development and financial success, and to encourage them to devote their best efforts to our business, thereby advancing our interests and the interests of our stockholders. By means of the 2016 Plan, we seek to retain the services of such eligible persons and to provide incentives for such persons to exert maximum efforts for our success and the success of our subsidiaries.
Description of the 2016 Equity Incentive Plan
The following is a summary description of the principal terms of the 2016 Plan and is qualified in its entirety by the full text of the 2016 Plan.
Administration. The 2016 Plan is administered by the Compensation Committee of our board of directors. The Compensation Committee is authorized to grant options to purchase shares of our common stock, stock appreciation rights, restricted stock, stock units, performance shares, performance units, incentive bonus awards, other cash-based awards and other stock-based awards. Stock options granted under the Motus G.I. Medical Technologies LTD Employee Share Option Plan that were outstanding as of the Share Exchange Transaction were assumed by the 2016 Plan and continue in effect in accordance with their terms, subject to appropriate adjustments to reflect the Share Exchange Transaction (the “Assumed Options”). The Compensation Committee also has authority to determine the terms and conditions of each award, prescribe, amend and rescind rules and regulations relating to the 2016 Plan, and amend the terms of awards in any manner not inconsistent with the 2016 Plan (provided that no amendment may adversely affect the rights of a participant without his or her consent), including authority to reduce or reprice the exercise price of outstanding options or stock appreciation rights. The Compensation Committee is permitted to delegate to officers and employees authority to grant options and other awards to employees (other than themselves), subject to applicable law and restrictions in the 2016 Plan. No award will be granted under the 2016 Plan on or after the ten year anniversary of the adoption of the 2016 Plan by our board of directors, but awards granted prior to the ten year anniversary may extend beyond that date.
Eligibility. Persons who are eligible to receive awards under the 2016 Plan include any person who is an employee, officer, director, consultant, advisor or other individual service provider of the Company or any subsidiary, or any person who is determined by the Compensation Committee to be a prospective employee, officer, director, consultant, advisor or other individual service provider of the Company or any subsidiary.
| 66 |
Shares Subject to the 2016 Plan. The aggregate number of shares of our common stock that are available for issuance in connection with options and awards granted under the 2016 Plan and Assumed Options is 2,011,656. Incentive stock options may, but need not be, granted with respect to all of the shares available for issuance under the 2016 Plan. If any award granted under the 2016 Plan payable in shares of our common stock is forfeited, cancelled, returned for failure to satisfy vesting requirements, is otherwise forfeited, otherwise terminates without payment being made, or if shares of our common stock are surrendered in full or partial payment of the exercise price or withheld to cover withholding taxes on options or other awards, the number of shares of our common stock as to which such option or award was forfeited, or which were surrendered or withheld, will be available for future grants under the 2016 Plan.
In addition, the 2016 Plan contains an “evergreen” provision allowing for an annual increase, on January 1 of each year during the term of the 2016 Plan, in the number of shares of our common stock available for issuance under the 2016 Plan. The annual increase in the number of shares shall be equal to six percent (6%) of the total number of shares of our common stock outstanding on December 31st of the preceding calendar year; provided, however, that our board of directors may act prior to the first day of any calendar year to provide that there shall be no increase such calendar year, or that the increase shall be a lesser number of shares of our common stock than would otherwise occur.
Terms and Conditions of Options. Options granted under the 2016 Plan may be either “incentive stock options” that are intended to meet the requirements of Section 422 of the Code or “nonqualified stock options” that do not meet the requirements of Section 422 of the Code. The Compensation Committee will determine the exercise price of options granted under the 2016 Plan. The exercise price of stock options may not be less than the fair market value per share of our common stock on the date of grant (or 110% of fair market value in the case of incentive stock options granted to a ten-percent stockholder).
If on the date of grant our common stock is listed on a stock exchange or national market system, the fair market value will generally be the closing sale price on the date of grant. If our common stock is not traded on a stock exchange or national market system on the date of grant, the fair market value will generally be the average of the closing bid and asked prices for our common stock on the date of grant. If no such prices are available, the fair market value shall be determined in good faith by the Compensation Committee based on the reasonable application of a reasonable valuation method. Notwithstanding the foregoing, if the date for which fair market value is determined is the date on which the final prospectus relating to an initial public offering of the Company is filed, the fair market value for such date will be the “Price to the Public” (or equivalent) set forth on the cover page for the final prospectus.
No option will be exercisable for more than ten years from the date of grant (five years in the case of an incentive stock option granted to a ten-percent stockholder). Options granted under the 2016 Plan will be exercisable at such time or times as the Compensation Committee prescribes at the time of grant. No employee may receive incentive stock options that first become exercisable in any calendar year in an amount exceeding $100,000. The Compensation Committee has authority, in its discretion, to permit a holder of a nonqualified stock option to exercise the option before it has otherwise become exercisable, in which case the shares of our common stock issued to the recipient will be restricted stock subject to vesting requirements analogous to those that applied to the option before exercise.
Generally, the exercise price of an option is payable (a) in cash or by certified bank check, (b) through delivery of shares of our common stock having a fair market value equal to the purchase price, or (c) such other method as approved by the Compensation Committee and set forth in an award agreement. The Compensation Committee is also authorized to establish a cashless exercise program and to permit the exercise price to be satisfied by reducing from the shares otherwise issuable upon exercise a number of shares having a fair market value equal to the exercise price.
| 67 |
No option will be transferrable other than by will or by the laws of descent and distribution, and during a recipient’s lifetime an option will be exercisable only by the recipient. However, the Compensation Committee is authorized to permit the holder of nonqualified stock options, share-settled stock appreciation rights, restricted stock, performance shares or other share-settled stock based awards to transfer the option, right or other award to immediate family members, to a trust for estate planning purposes, or by gift to charitable institutions. The Compensation Committee has the authority to determine the extent to which a holder of a stock option may exercise the option following termination of service with us.
Stock Appreciation Rights. The Compensation Committee is authorized to grant stock appreciation rights (“SARs”) independent of or in connection with an option. The Compensation Committee is also authorized to determine the other terms applicable to SARs. The base price of a SAR will be determined by the Compensation Committee, but will not be less than 100% of the fair market value of a share of our common stock on the date of grant. The maximum term of any SAR granted under the 2016 Plan will be ten years from the date of grant. Generally, each SAR will entitle a participant upon exercise to an amount equal to:
| ● | the excess of the fair market value on the exercise date of one share of our common stock over the base price, multiplied by | |
| ● | the number of shares of our common stock as to which the SAR is exercised. |
Payment may be made in shares of our common stock, in cash, or partly in shares of our common stock and partly in cash, all as determined by the Compensation Committee.
Restricted Stock and Stock Units. The Compensation Committee is authorized to award restricted common stock and/or stock units under the 2016 Plan. Restricted stock awards consist of shares of stock that are transferred to a participant subject to restrictions that may result in forfeiture if specified conditions are not satisfied. Stock units confer the right to receive shares of our common stock, cash, or a combination of shares and cash, at a future date upon or following the attainment of such conditions as may be specified by the Compensation Committee. The Compensation Committee is authorized to determine the restrictions and conditions applicable to each award of restricted stock or stock units, which may include performance-based conditions. The 2016 Plan provides that dividends with respect to restricted stock may be paid to the holder of the shares as and when dividends are paid to stockholders or at the time that the restricted stock vests, as determined by the Compensation Committee. Dividend equivalent amounts under the 2016 Plan may also be paid with respect to stock units, and are subject to the same restrictions on transferability as the stock units with respect to which they were paid. Unless the Compensation Committee determines otherwise, holders of restricted stock have the right to vote the shares.
Performance Shares and Performance Units. The Compensation Committee is authorized to award performance shares and/or performance units under the 2016 Plan. Performance shares and performance units are awards, denominated in either shares or U.S. dollars, which are earned during a specified performance period subject to the attainment of performance criteria, as established by the Compensation Committee. The Compensation Committee has the authority to determine the restrictions and conditions applicable to each award of performance shares and performance units.
Incentive Bonus Awards. The Compensation Committee is authorized to award incentive bonus awards payable in cash or shares of our common stock, as set forth in an award agreement. The Compensation Committee has the authority to determine the terms and conditions applicable to each incentive bonus award.
Other Stock-Based and Cash-Based Awards. The Compensation Committee is authorized to award other types of equity-based or cash-based awards under the 2016 Plan, including the grant or offer for sale of shares of our common stock that do not have vesting requirements and the right to receive one or more cash payments subject to satisfaction of such conditions as the Compensation Committee may impose.
| 68 |
Section 162(m) Compliance. If stock or cash-based awards are intended to satisfy the conditions for deductibility under Section 162(m) of the Code as “performance-based compensation,” the performance criteria will be selected from among the following performance criteria, which may be applied to our Company as a whole, or to any subsidiary or any division or operating unit thereof: (a) pre-tax income; (b) after-tax income; (c) net income; (d) operating income or profit; (e) cash flow, free cash flow, cash flow return on investment (discounted or otherwise), net cash provided by operations, or cash flow in excess of cost of capital; (f) earnings per share (basic or diluted); (g) return on equity; (h) returns on sales or revenues; (i) return on invested capital or assets (gross or net); (j) cash, funds or earnings available for distribution; (k) appreciation in the fair market value of our common stock; (l) operating expenses; (m) implementation or completion of critical projects or processes; (n) return on investment; (o) total return to stockholders (meaning the aggregate common stock price appreciation and dividends paid (assuming full reinvestment of dividends) during the applicable period); (p) net earnings growth; (q) return measures (including but not limited to return on assets, capital, equity, or sales); (r) increase in revenues; (s) the Company’s published ranking against its peer group of companies based on total stockholder return; (t) net earnings; (u) changes (or the absence of changes) in the per share price of the Company’s common stock; (v) preclinical, clinical or regulatory milestones; (w) earnings before or after any one or more of the following items: interest, taxes, depreciation or amortization, as reflected in the Company’s financial reports for the applicable period; (x) total revenue growth (meaning the increase in total revenues after the date of grant of an award and during the applicable period, as reflected in the Company’s financial reports for the applicable period); (y) economic value created; (z) operating margin or profit margin; (aa) share price or total shareholder return; (bb) cost targets, reductions and savings, productivity and efficiencies; (cc) strategic business criteria, consisting of one or more objectives based on meeting objectively determinable criteria: specified market penetration, geographic business expansion, investor satisfaction, employee satisfaction, human resources management, supervision of litigation, information technology, and goals relating to acquisitions, divestitures, joint ventures and similar transactions, and budget comparisons; (dd) objectively determinable personal or professional objectives, including any of the following performance goals: the implementation of policies and plans, the negotiation of transactions, the development of long term business goals, formation of joint ventures, research or development collaborations, and the completion of other corporate transactions; and (ee) any combination of, or a specified increase or improvement in, any of the foregoing.
At the end of the performance period established in connection with any award, the Compensation Committee will determine the extent to which the performance goal or goals established for such award have been attained, and will determine, on that basis, the shares or, if applicable, the cash or other property that has been earned and as to which payment will be made. The Compensation Committee will certify in writing the extent to which it has determined that the performance goal or goals established by it for such award have been attained.
The maximum number of shares of our common stock with respect to which any one participant may be granted stock options or stock appreciation rights during any calendar year is 1,500,000 shares. With respect to awards intended to be exempt from the deductibility limitation in Section 162(m) of the Code (other than stock options and stock appreciation rights), (i) the maximum number of shares of our common stock that may be paid to any one individual in respect of any calendar year if the applicable performance goals are attained is 1,500,000 shares, and (ii) the maximum cash amount that may be paid to any one participant in respect of any calendar year if the applicable performance goals are attained is $1,000,000. Each such maximum number of shares is subject to adjustment in the event of a recapitalization, stock split, merger, reorganization or similar corporate change affecting our common stock. If the performance period for certain performance goals spans more than one calendar year, the shares or cash paid in respect of each calendar year is determined by pro rating the shares or cash paid for the performance period based on the number of performance period days that fall in each respective calendar year.
Effect of Certain Corporate Transactions. The Compensation Committee has the authority to provide, at the time of the grant of an award, for the effect of a change in control (as defined in the 2016 Plan) on any award, including (i) accelerating or extending the time periods for exercising, vesting in, or realizing gain from any award, (ii) eliminating or modifying the performance or other conditions of an award, (iii) providing for the cash settlement of an award for an equivalent cash value, as determined by the Compensation Committee, or (iv) such other modification or adjustment to an award as the Compensation Committee deems appropriate to maintain and protect the rights and interests of participants following a change in control. The Compensation Committee has the authority, in its discretion and without the need for the consent of any recipient of an award, to also take one or more of the following actions contingent upon the occurrence of a change in control: (a) cause any or all outstanding options and stock appreciation rights to become immediately exercisable, in whole or in part; (b) cause any other awards to become non-forfeitable, in whole or in part; (c) cancel any option or stock appreciation right in exchange for a substitute option; (d) cancel any award of restricted stock, stock units, performance shares or performance units in exchange for a similar award of the capital stock of any successor corporation; (e) redeem any restricted stock for cash and/or other substitute consideration with a value equal to the fair market value of an unrestricted share of our common stock on the date of the change in control; (f) cancel any option or stock appreciation right in exchange for cash and/or other substitute consideration based on the value of our common stock on the date of the change in control, and cancel any option or stock appreciation right without any payment if its exercise price exceeds the value of our common stock on the date of the change in control; or (g) make such other modifications, adjustments or amendments to outstanding awards as the Compensation Committee deems necessary or appropriate.
| 69 |
Amendment, Termination. The Compensation Committee has the authority to amend the terms of awards in any manner not inconsistent with the 2016 Plan, provided that no amendment shall adversely affect the rights of a participant with respect to an outstanding award without the participant’s consent. In addition, our board of directors has the authority, at any time, to amend, suspend, or terminate the 2016 Plan, provided that (i) no such amendment, suspension or termination materially and adversely affects the rights of any participant under any outstanding award without the consent of such participant and (ii) to the extent necessary to comply with any applicable law, regulation, or stock exchange rule, the 2016 Plan requires us to obtain stockholder consent. Stockholder approval is required for any plan amendment that increases the number of shares of our common stock available for issuance under the 2016 Plan or changes the persons or classes of persons eligible to receive awards.
Tax Withholding
As and when appropriate, we shall have the right to require each optionee purchasing shares of our common stock and each grantee receiving an award of shares of our common stock under the 2016 Plan to pay any federal, state or local taxes required by law to be withheld.
Option Grants and Stock Awards
The grant of options and other awards under the 2016 Plan is discretionary and we cannot determine now the specific number or type of options or awards to be granted in the future to any particular person or group.
Israeli Aspects of the 2016 Plan
The 2016 Israeli Sub-Plan (the “Sub-Plan”) provides for the grant of awards pursuant to the Israeli Income Tax Ordinance (New Version), 1960, as amended (the “Israeli Tax Ordinance”): awards granted pursuant to (i) Section 102 of the Israeli Tax Ordinance (“Section 102 Awards”) and (ii) Section 3(i) of the Israeli Tax Ordinance (“Section 3(i) Awards”). The 2016 Plan and the Sub-Plan provide, subject to applicable law, that Section 102 Awards may be granted only to Israeli employees, officers and directors (excluding Controlling Shareholders as defined by the Israeli Tax Ordinance1) and Section 3(i) Awards (which does not provide for similar tax benefits) may be granted to Israeli non-employees including consultants, service providers and Controlling Shareholders (as defined by the Israeli Tax Ordinance), in each case, of our company or any subsidiary. The 2016 Plan and the Sub-Plan were submitted for the approval of the Israeli Tax Authority (the “ITA”), as required by applicable law.
Section 102 of the Israeli Tax Ordinance includes two alternatives for tax treatment involving the issuance of options or shares to a trustee for the benefit of the grantees, which are referred to as the capital gains track and the ordinary income track, and also includes an additional alternative for the issuance of options or shares issued directly to the grantee. Under the Sub- Plan, each Section 102 Award designates that such award be granted under the capital gains track or the ordinary income track. We cannot select both tracks simultaneously for Section 102 Awards and the election of the type of track shall apply to all Section 102 Awards awarded under the Sub-Plan (unless the election is changed pursuant to the provisions of the Israeli Tax Ordinance).
The Assumed Options granted to employees under the Motus G.I. Medical Technologies Ltd. Employee Share Option Plan, were granted under Section 102(b)(2) of the Israeli Tax Ordinance, which permits the issuance to a trustee under the “capital gains track.” In order to comply with the terms of the “capital gains track”, all options granted under a specific plan and subject to the provisions of Section 102 of the Israeli Tax Ordinance, as well as the shares issued upon exercise of such options and other shares received subsequently following any realization of rights with respect to such options, such as share dividends and share splits, must be registered in the name of a trustee selected by the board of directors and held in trust for the benefit of the relevant employee, director or officer for a period of two years from the date of grant and deposit with such trustee. However, under this track, the “employing company” (within the meaning of Section 102(a) of the Israeli Tax Ordinance) is not allowed to deduct an expense with respect to the issuance of the options or shares.
Indemnification Agreements
We have entered into Indemnification Agreements with certain of our current directors and executive officers. The Indemnification Agreements provide for indemnification against expenses, judgments, fines and penalties actually and reasonably incurred by an indemnitee in connection with threatened, pending or completed actions, suits or other proceedings, subject to certain limitations. The Indemnification Agreements also provide for the advancement of expenses in connection with a proceeding prior to a final, nonappealable judgment or other adjudication, provided that the indemnitee provides an undertaking to repay to us any amounts advanced if the indemnitee is ultimately found not to be entitled to indemnification by us. The Indemnification Agreement sets forth procedures for making and responding to a request for indemnification or advancement of expenses, as well as dispute resolution procedures that will apply to any dispute between us and an indemnitee arising under the Indemnification Agreements.
1 Controlling Shareholder is defined in the Israeli Tax Ordinance as any person who holds, directly or indirectly, individually or together with any of his relatives (as defined in the Israeli Tax Ordinance), any of the following: (i) at least 10% of the outstanding share capital or voting rights of the company; (ii) the right to hold or acquire at least 10% of the outstanding share capital or voting rights of the company; (iii) the right to receive at least 10% of the company’s profits; or (iv) the right to appoint a director of the company.
| 70 |
The following table sets forth information regarding the beneficial ownership of our common stock as of the date of this prospectus by:
| ● | each of our stockholders who is known by us to beneficially own 5% or more of our common stock; | |
| ● | each of our named executive officers; | |
| ● | each of our directors; and | |
| ● | all of our directors and current officers as a group. |
Beneficial ownership is determined based on the rules and regulations of the SEC. A person has beneficial ownership of shares if such individual has the power to vote and/or dispose of shares. This power may be sole or shared and direct or indirect. In computing the number of shares beneficially owned by a person and the percentage ownership of that person, shares of our common stock that are subject to options or warrants held by that person and exercisable as of, or within 60 days of, September 30, 2017 are counted as outstanding. These shares, however, are not counted as outstanding for the purposes of computing the percentage ownership of any other person(s). Except as may be indicated in the footnotes to this table and pursuant to applicable community property laws, each person named in the table has sole voting and dispositive power with respect to the shares of our common stock set forth opposite that person’s name. Unless indicated below, the address of each individual listed below is c/o Motus GI Holdings, Inc., 1301 East Broward Boulevard, 3rd Floor, Ft. Lauderdale, FL 33301.
Applicable percentage ownership in the following table is based on 10,491,841 shares of our common stock outstanding as of September 30, 2017. Beneficial ownership representing less than 1% is denoted with an asterisk (*).
| Name of Beneficial Owner | Number of Shares Beneficially Owned | Percentage of Shares Beneficially Owned | ||||||||||||||
| Before Offering | After Offering | Before Offering | After Offering | |||||||||||||
| Officers and Directors | ||||||||||||||||
| Mark Pomeranz (1) | 329,144 | 3.04 | % | |||||||||||||
| David Hochman (2)(4)(5)(6) | 2,499,182 | 23.07 | % | |||||||||||||
| Darren Sherman (3)(4)(5)(6) | 2,504,544 | 23.11 | % | |||||||||||||
| Gary Jacobs (7)(8) | 796,862 | 7.5 | % | |||||||||||||
| Samuel Nussbaum (9) | - | * | ||||||||||||||
| Shervin Korangy (10) | - | * | ||||||||||||||
| Andrew Taylor (11) | - | * | ||||||||||||||
| Directors and Officers as a Group (6 persons) | 3,633,550 | 32.16 | % | |||||||||||||
| 5% Stockholders | ||||||||||||||||
| Ascent Biomedical Ventures II, L.P. (12) | 1,751,947 | 16.23 | % | |||||||||||||
| Ascent Biomedical Ventures Synecor, L.P. (13) | 640,039 | 6.07 | % | |||||||||||||
| ABV, LLC (12)(13) | 2,391,986 | 22.05 | % | |||||||||||||
| Orchestra Medical Ventures II, L.P. (4) | 1,183,726 | 11.06 | % | |||||||||||||
| Orchestra MOTUS Co-Investment Partners, LLC (5) | 1,229,104 | 11.57 | % | |||||||||||||
| Orchestra Medical Ventures II GP, LLC (4)(5)(6) | 2,496,182 | 23.04 | % | |||||||||||||
| Jacobs Investment Company LLC (8) | 792,762 | 7.46 | % | |||||||||||||
| Perceptive Life Sciences Master Fund Ltd. (14) | 1,866,541 | 17.26 | % | |||||||||||||
| Perceptive Advisors LLC (14) | 1,866,541 | 17.26 | % | |||||||||||||
| Brian Eliot Peierls (15)(17) | 654,217 | 6.11 | % | |||||||||||||
| E. Jeffrey Peierls (16)(17) | 677,889 | 6.32 | % | |||||||||||||
| 71 |
* Less than 1%
| 1. | Includes 329,003 shares of our common stock issuable upon the exercise of stock options that are exercisable within sixty days of September 30, 2017. Does not include 249,348 shares of our common stock issuable upon the exercise of stock options that are not exercisable within sixty days of September 30, 2017. |
| 2. | Does not include 175,000 shares of our common stock issuable upon the exercise of stock options that are not exercisable within sixty days of September 30, 2017. |
| 3. | Does not include 100,000 shares of our common stock issuable upon the exercise of stock options that are not exercisable within sixty days of September 30, 2017. |
| 4. | Includes (i) 975,140 shares of common stock (ii) 99,748 shares of common stock issuable upon the conversion of Series A Convertible Preferred Stock and (iii) 108,838 shares of common stock issuable upon exercise of Exchange Warrants, held by Orchestra Medical Ventures II, L.P. The managing members of Orchestra Medical Ventures II GP, LLC, David Hochman and Darren Sherman, exercise sole dispositive and voting power over the shares owned by Orchestra Medical Ventures II, L.P. |
| 5. | Includes (i) 1,094,930 shares of common stock (ii) 65,038 shares of common stock issuable upon the conversion of Series A Convertible Preferred Stock and (iii) 69,136 shares of common stock issuable upon exercise of Exchange Warrants, held by Orchestra MOTUS Co-Investment Partners, LLC. The managing members of Orchestra Medical Ventures II GP, LLC, David Hochman and Darren Sherman, exercise sole dispositive and voting power over the shares owned by Orchestra MOTUS Co-Investment Partners, LLC. |
| 6. | Includes (i) 83,352 shares of common stock held by Orchestra Medical Ventures II Reserve, L.P. The managing members of Orchestra Medical Ventures II GP, LLC, David Hochman and Darren Sherman, exercise sole dispositive and voting power over the shares owned by Orchestra Medical Ventures II Reserve, L.P. |
| 7. | Does not include 92,500 shares of our common stock issuable upon the exercise of stock options that are not exercisable within sixty days of September 30, 2017. |
| 72 |
| 8. | Includes (i) 660,567 shares of common stock (ii) 63,289 shares of common stock issuable upon the conversion of Series A Convertible Preferred Stock and (iii) 68,906 shares of common stock issuable upon exercise of Exchange Warrants, held by Jacobs Investment Company LLC. The managing member of Jacobs Investment Company LLC, Gary Jacobs, exercises sole dispositive and voting power over the shares owned by Jacobs Investment Company LLC. |
| 9. | Does not include 50,000 shares of our common stock issuable upon the exercise of stock options that are not exercisable within sixty days of September 30, 2017. |
| 10. | Does not include 65,000 shares of our common stock issuable upon the exercise of stock options that are not exercisable within sixty days of September 30, 2017. |
| 11. | Does not include 240,000 shares of our common stock issuable upon the exercise of stock options that are not exercisable within sixty days of September 30, 2017. |
| 12. | Includes (i) 1,450,861 shares of common stock (ii) 144,352 shares of common stock issuable upon the conversion of Series A Convertible Preferred Stock and (iii) 156,734 shares of common stock issuable upon exercise of Exchange Warrants, held by Ascent Biomedical Ventures II, L.P. The managing members of ABV, LLC, Geoffrey W. Smith and Steve Hochberg, exercise sole dispositive and voting power over the shares owned by Ascent Biomedical Ventures II, L.P. The principal address for the entities affiliated with ABV, LLC is 60 East 42nd Street, New York, NY 10165. |
| 13. | Includes (i) 586,365 shares of common stock (ii) 26,241 shares of common stock issuable upon the conversion of Series A Convertible Preferred Stock and (iii) 27,433 shares of common stock issuable upon exercise of Exchange Warrants, held by Ascent Biomedical Ventures Synecor, L.P. The managing members of ABV, LLC, Geoffrey W. Smith and Steve Hochberg, exercise sole dispositive and voting power over the shares owned by Ascent Biomedical Ventures Synecor, L.P. The principal address for the entities affiliated with ABV, LLC is 60 East 42nd Street, New York, NY 10165. |
| 14. | Includes (i) 1,544,155 shares of common stock (ii) 256,386 shares of common stock issuable upon the conversion of Series A Convertible Preferred Stock and (iii) 66,000 shares of common stock issuable upon exercise of Exchange Warrants, held by Perceptive Life Sciences Master Fund Ltd. The managing member of Perceptive Advisors LLC, Mr. Joseph Edelman, exercises sole dispositive and voting power over the shares owned by Perceptive Life Sciences Master Fund Ltd. The principal address for the entities affiliated with Perceptive Advisors LLC is 51 Astor Place, 10th floor New York, NY 10003. |
| 15. | Includes (i) 35,257 shares of common stock (ii) 9,086 shares of common stock issuable upon the conversion of Series A Convertible Preferred Stock and (iii) 7,920 shares of common stock issuable upon exercise of Exchange Warrants, held by Brian Eliot Peierls. The principal address for Brian Eliot Peierls is 3017 McCurdy St., Austin TX 78723. |
| 16. | Includes (i) 50,542 shares of common stock (ii) 13,381 shares of common stock issuable upon the conversion of Series A Convertible Preferred Stock and (iii) 12,012 shares of common stock issuable upon exercise of Exchange Warrants, held by E. Jeffrey Peierls. The principal address for E. Jeffrey Peierls is 73 South Holman Way, Golden, CO 80401. |
| 17. | Includes (i) an aggregate of 400,185 shares of common stock (ii) an aggregate of 106,201 shares of common stock issuable upon the conversion of Series A Convertible Preferred Stock and (iii) 95,568 shares of common stock issuable upon exercise of Exchange Warrants, held by The Peierls Bypass Trust, UD E.F. Peierls for Brian E. Peierls, UD E.F. Peierls for E. Jeffrey Peierls, UD E.S. Peierls for E.F. Peierls et al, UD J.N. Peierls for Brian Eliot Peierls, UD J.N. Peierls for E. Jeffrey Peierls, UW E.S. Peierls for Brian E. Peierls - Accumulation, UW E.S. Peierls for E. Jeffrey Peierls - Accumulation, UW J.N. Peierls for Brian E. Peierls, UW J.N. Peierls for E. Jeffrey Peierls (collectively, the “Peierls Trusts”) and The Peierls Foundation, Inc. and UD Ethel F. Peierls Charitable Lead Trust (collectively, the “Peierls Entities”). E. Jeffrey Peierls and Brian Eliot Peierls share dispositive and voting power over the shares owned by the Peierls Entities. E. Jeffrey Peierls expressly disclaims beneficial ownership with respect to the shares owned by the Peierls Entities, as he has no pecuniary interest therein. The principal address for the Peierls Trusts is c/o The Northern Trust Company of Delaware, 1313 N. Market Street, Ste 5300, Wilmington, DE 19801. The principal address for the Peierls Entities is 73 South Holman Way, Golden, CO 80401. |
| 73 |
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
Other than compensation arrangements for our named executive officers and directors, we describe below each transaction or series of similar transactions, since January 1, 2014, to which we were a party or will be a party, in which:
| ● | the amounts involved exceeded or will exceed the lesser of (i) $120,000 or (ii) 1% of the average total assets of the Company at year end for the last two completed fiscal years; and | |
| ● | any of our directors, executive officers, promoters or holders of more than 5% of our capital stock, or any member of the immediate family of the foregoing persons, had or will have a direct or indirect material interest. |
Compensation arrangements for our named executive officers and directors are described in the section entitled “Executive Compensation.” Mark Pomeranz and David Hochman are our founders and, therefore, may be considered promoters, as that term is defined in Rule 405 of Regulation C of the Securities Act.
Convertible Note, Convertible Warrant, and 2017 Private Placement - Related Party Participation
From August 2015 through November 2016, Orchestra Medical Ventures II, L.P., an affiliate of Orchestra Medical Ventures, LLC (an investment firm in which David Hochman, Chairman of the Board, and Darren Sherman, one of our Directors, serve as Managing Partners) purchased Convertible Notes in an aggregate principal amount of $1,649,062. On December 22, 2016, Orchestra Medical Ventures II, L.P. exchanged its Convertible Notes, together with accrued and unpaid interest thereon calculated through December 22, 2016 at a rate of 10% per annum, for Units sold in the 2017 Private Placement at a price of $4.50 per Unit resulting in the receipt of (i) 299,244 shares of our common stock and (ii) 99,748 shares of our Series A Convertible Preferred Stock. In addition, Orchestra Medical Ventures II, L.P. received five (5) year warrants to purchase an aggregate of 108,838 shares of our common stock at an exercise price of $5.00 per share in an amount equal to thirty-three percent (33%) of the principal amount of such Convertible Note divided by $5.00 (the “Exchange Warrants”).
In addition, from August 2015 through November 2016, Orchestra MOTUS Co-Investment Partners, LLC, an affiliate of Orchestra Medical Ventures, LLC (an investment firm in which David Hochman, Chairman of the Board, and Darren Sherman, one of our Directors, serve as Managing Partners) purchased Convertible Notes in an aggregate principal amount of $1,047,511. On December 22, 2016, Orchestra MOTUS Co-Investment Partners, LLC exchanged its Convertible Notes, together with accrued and unpaid interest thereon calculated through December 22, 2016 at a rate of 10% per annum, for Units sold in the 2017 Private Placement at a price of $4.50 per Unit resulting in the receipt of (i) 195,114 shares of our common stock and (ii) 65,038 shares of our Series A Convertible Preferred Stock. In addition, Orchestra MOTUS Co-Investment Partners, LLC received Exchange Warrants to purchase an aggregate of 69,136 shares of our common stock.
From June 2015 through November 2016, Jacobs Investment Company, LLC, an investment firm in which Gary Jacobs, one of our Directors, serves as Founder and Managing Director, purchased Convertible Notes in an aggregate principal amount of $1,044,032. On December 22, 2016, Jacobs Investment Company, LLC exchanged its Convertible Notes, together with accrued and unpaid interest thereon calculated through December 22, 2016 at a rate of 10% per annum, for Units sold in the 2017 Private Placement at a price of $4.50 per Unit resulting in the receipt of (i) 189,865 shares of our common stock and (ii) 63,289 shares of our Series A Convertible Preferred Stock. In addition, Jacobs Investment Company, LLC received Exchange Warrants to purchase an aggregate of 68,906 shares of our common stock.
From June 2015 through August 2016, Ascent Biomedical Ventures II, L.P., and from July 2015 through October 2015, Ascent Biomedical Ventures Synecor, L.P. (collectively, the “Ascent Entities”) purchased Convertible Notes in an aggregate principal amount of $2,790,412 (the “Ascent Convertible Notes”). ABV, LLC, a beneficial owner of more than five percent of our common stock, is the beneficial owner of the securities held by the Ascent Entities. On December 22, 2016, the Ascent Entities exchanged the Ascent Convertible Notes, together with accrued and unpaid interest thereon calculated through December 22, 2016 at a rate of 10% per annum, for Units sold in the 2017 Private Placement at a price of $4.50 per Unit resulting in the receipt of (i) 511,776 shares of our common stock and (ii) 170,593 shares of our Series A Convertible Preferred Stock. In addition, the Ascent Entities received Exchange Warrants to purchase an aggregate of 184,167 shares of our common stock.
| 74 |
On October 27, 2016, Perceptive Life Sciences Master Fund Ltd., and on October 28, 2016, Titan Perc, Ltd. (collectively, the “Perceptive Entities”) purchased Convertible Notes in an aggregate principal amount of $1,000,000 (the “Perceptive Convertible Notes”). Perceptive Advisors LLC, a beneficial owner of more than five percent of our common stock, is the beneficial owner of the securities held by the Perceptive Entities. On December 22, 2016, the Perceptive Entities exchanged the Perceptive Convertible Notes, together with accrued and unpaid interest thereon calculated through December 22, 2016 at a rate of 10% per annum, for Units sold in the 2017 Private Placement at a price of $4.50 per Unit resulting in the receipt of (i) 169,155 shares of our common stock and (ii) 56,386 shares of our Series A Convertible Preferred Stock. In addition, the Perceptive Entities received Exchange Warrants to purchase an aggregate of 66,000 shares of our common stock. Additionally, the Perceptive entities purchased an aggregate of 800,000 Units in our 2017 Private Placement, at a purchase price of $5.00 per Unit, comprised of an aggregate of (i) 600,000 shares of our common stock and (ii) 200,000 shares of our Series A Convertible Preferred Stock.
From January 2016 through November 2016 the Peierls Trusts and the Peierls Entities purchased Convertible Notes in an aggregate principal amount of $1,448,000 (the “Peierls Convertible Notes”). Brian Eliot Peierls and E. Jeffrey Peierls, each beneficial owners of more than five percent of our common stock, are the beneficial owners of the securities held by the Peierls Trusts and the Peierls Entities. On December 22, 2016, the Peierls Trusts and the Peierls Entities exchanged the Peierls Convertible Notes, together with accrued and unpaid interest thereon calculated through December 22, 2016 at a rate of 10% per annum, for Units sold in the 2017 Private Placement at a price of $4.50 per Unit resulting in the receipt of (i) 257,385 shares of our common stock and (ii) 85,801 shares of our Series A Convertible Preferred Stock. In addition, the Peierls Trusts and the Peierls Entities received Exchange Warrants to purchase an aggregate of 95,568 shares of our common stock. Additionally, the Peierls Trusts and the Peierls Entities purchased an aggregate of 100,000 Units in our 2017 Private Placement, at a purchase price of $5.00 per Unit, comprised of an aggregate of (i) 75,000 shares of our common stock and (ii) 25,000 shares of our Series A Convertible Preferred Stock.
On October 27, 2016, AKS Family Partners, LP, a beneficial owner of more than five percent of our common stock prior to the Initial Closing, purchased a Convertible Note in an aggregate principal amount of $250,000. On December 22, 2016, AKS Family Partners, LP exchanged its Convertible Note, together with accrued and unpaid interest thereon calculated through December 22, 2016 at a rate of 10% per annum, for Units sold in the 2017 Private Placement at a price of $4.50 per Unit resulting in the receipt of (i) 42,290 shares of our common stock and (ii) 14,097 shares of our Series A Convertible Preferred Stock. In addition, AKS Family Partners, LP received Exchange Warrants to purchase an aggregate of 16,500 shares of our common stock. As a result of the Initial Closing, AKS Family Partners, LP was no longer a beneficial owner of more than five percent of our common stock.
Share Exchange Transaction
Effective on December 1, 2016, Opco, and the Opco Stockholders, entered into the Share Exchange Agreement with us. Pursuant to the terms of the Share Exchange Agreement, the Opco Stockholders sold to us, and we acquired, all of the issued and outstanding shares of capital stock of Opco and Opco became our wholly owned subsidiary.
At the closing of the Share Exchange Transaction, on December 22, 2016, (i) Orchestra Medical Ventures II, L.P. and Orchestra MOTUS Co-Investment Partners, LLC, both affiliates of Orchestra Medical Ventures, LLC (an investment firm in which David Hochman, Chairman of the Board, and Darren Sherman, one of our Directors, serve as Managing Partners) sold to us, and we acquired, all of the capital stock of Opco held by Orchestra Medical Ventures II, L.P. and Orchestra MOTUS Co-Investment Partners, LLC in exchange for 670,800 and 899,816 shares of our Common Stock, respectively, (ii) Jacobs Investment Company, LLC, an investment firm in which Gary Jacobs, one of our Directors, serves as Founder and Managing Director, sold to us, and we acquired, all of the capital stock of Opco held by Jacobs Investment Company, LLC in exchange for 474,802 shares of our Common Stock, and (iii) ABV, LLC, a beneficial owner of more than five percent of our common stock, through the Ascent Entities, sold to us, and we acquired, all of the capital stock of Opco held by the Ascent Entities in exchange for an aggregate of 1,520,353 shares of our Common Stock.
| 75 |
See “Prospectus Summary – Formation of Holdings – The Share Exchange Transaction” for a description of the Share Exchange Transaction and the terms of the Share Exchange Agreement.
Participation in this Offering
Certain of our existing stockholders, including stockholders affiliated with certain of our directors, have indicated an interest in purchasing, including and its affiliates, which have indicated an interest in purchasing up to $ million of such shares of our common stock at the initial public offering price, and its affiliates, which have indicated an interest in purchasing up to $ million of such shares of our common stock at the initial public offering price, and its affiliates, which have indicated an interest in purchasing up to $ million of such shares of our common stock at the initial public offering price and and its affiliates, which have indicated an interest in purchasing up to $ million of such shares of our common stock at the initial public offering price. However, because indications of interest are not binding agreements or commitments to purchase, the underwriters could determine to sell more, less or no shares to any of these potential investors and any of these potential investors could determine to purchase more, less or no shares in this offering. Any shares purchased by our existing stockholders will be subject to lock-up restrictions described in “Shares Eligible for Future Sale.”
Indemnification Agreements
In 2017 we entered into indemnification agreements with certain of our directors and officers. For more information, see the description of the indemnification agreements under “Management and Board of Directors - Limitation of Directors Liability and Indemnification.”
Policies and Procedures for Related Party Transactions
Our board of directors has adopted a policy that our executive officers, directors, nominees for election as a director, beneficial owners of more than 5% of any class of our common stock, any members of the immediate family of any of the foregoing persons and any firms, corporations or other entities in which any of the foregoing persons is employed or is a partner or principal or in a similar position or in which such person has a 5% or greater beneficial ownership interest (collectively “related parties”), are not permitted to enter into a transaction with us without the prior consent of our board of directors acting through the audit committee or, in certain circumstances, the chairman of the audit committee. Any request for us to enter into a transaction with a related party, in which the amount involved exceeds $100,000 and such related party would have a direct or indirect interest must first be presented to our audit committee, or in certain circumstances the chairman of our audit committee, for review, consideration and approval. In approving or rejecting any such proposal, our audit committee, or the chairman of our audit committee, is to consider the material facts of the transaction, including, but not limited to, whether the transaction is on terms no less favorable than terms generally available to an unaffiliated third party under the same or similar circumstances, the extent of the benefits to us, the availability of other sources of comparable products or services and the extent of the related party’s interest in the transaction.
| 76 |
Our current certificate of incorporation, as amended, authorizes us to issue:
| ● | 50,000,000 shares of common stock, par value $0.0001 per share; and | |
| ● | 10,000,000 shares of preferred stock, par value $0.0001 per share. |
As of September 30, 2017 there were 10,491,841 shares of our common stock outstanding, held of record by 292 stockholders, and 1,581,128 shares of preferred stock outstanding.
The following statements are summaries only of provisions of our authorized capital stock and are qualified in their entirety by our certificate of incorporation, as amended. You should review these documents for a description of the rights, restrictions and obligations relating to our capital stock. Copies of our certificate of incorporation may be obtained from the Company upon written request.
Common stock
Voting. The holders of our common stock are entitled to one vote for each share held of record on all matters on which the holders are entitled to vote (or consent to). When a quorum is present at any meeting of stockholders, any matter before any such meeting (other than an election of a director or directors) shall be decided by a majority of the votes properly cast on such matter, except where a different vote is required by law, by the rules or regulations of any stock exchange applicable to the Corporation, or pursuant to any regulation applicable to the Corporation or its securities, in which case, such different vote shall apply. A majority in voting power of the shares entitled to vote at the meeting, present in person or represented by proxy, shall constitute a quorum at any meeting of stockholders.
Dividends. The holders of our common stock are entitled to receive, ratably, dividends only if, when and as declared by our board of directors out of funds legally available therefor and after provision is made for each class of capital stock having preference over our common stock.
Liquidation Rights. In the event of our liquidation, dissolution or winding-up, the holders of our common stock are entitled to share, ratably, in all assets remaining available for distribution after payment of all liabilities and after provision is made for each class of capital stock having preference over our common stock.
Conversion Rights. The holders of our common stock have no conversion rights.
Preemptive and Similar Rights. The holders of our common stock have no preemptive or similar rights.
Redemption/Put Rights. There are no redemption or sinking fund provisions applicable to our common stock. All of the outstanding shares of our common stock are fully-paid and non-assessable.
Transfer Restrictions. Shares of our common stock are subject to transfer restrictions. Holders of our common stock may not transfer their securities unless (a) a registration statement is in effect under the Securities Act covering the proposed transfer and such transfer is made in accordance with such registration statement or (b) the securities are transferred in a transaction exempt from the registration requirements of the Securities Act and any related requirements imposed by applicable state securities laws. In the case of any transfer permitted under clause (b), the holder must notify us in writing of the proposed transfer and furnish us with an opinion of counsel, reasonably satisfactory to us, that the transfer will not require registration under the Securities Act or any applicable state securities laws. Each certificate representing a security contains a legend referring to this restriction on transfer and any legends required by state securities laws. The securities are also subject to other restrictions on transfer as provided in the Registration Rights Agreement, described below.
| 77 |
Preferred Stock
We are authorized to issue up to 10,000,000 shares of “blank check” preferred stock, par value $0.0001 per share, with such designations, rights, and preferences as may be determined from time to time by our board of directors. Accordingly, our board of directors is empowered, without stockholder approval, to issue preferred stock with dividend, liquidation, conversion, voting, or other rights that could adversely affect the voting power or other rights of the holders of our common stock. The issuance of preferred stock could have the effect of restricting dividends on our common stock, diluting the voting power of our common stock, impairing the liquidation rights of our common stock, or delaying or preventing a change in control of our company, all without further action by our Series A Convertible Preferred Stock stockholders.
In connection with the 2017 Private Placement, our board of directors created out of the authorized and unissued shares of our preferred stock, a series of preferred stock comprised of up to 2,000,000 shares of Series A Convertible Preferred Stock, of which 1,581,128 are currently issued and outstanding.
Rank. The Series A Convertible Preferred Stock rank above all other classes of stock outstanding as of the date hereof with respect to dividend rights and liquidation preferences.
Liquidation. Upon any dissolution, liquidation or winding up, whether voluntary or involuntary, holders of Series A Convertible Preferred Stock are entitled to (i) first receive distributions out of our assets in an amount per share equal to $5.00 (the “Stated Value”), whether capital or surplus before any distributions shall be made on any shares of our common stock and (ii) second, on an as-converted basis alongside our common stock.
Conversion. Upon the earlier of (i) December 22, 2019, without any action on the part of the holder, or (ii) notice by the Company to the Holders that the Company has elected to convert all outstanding Series A Convertible Preferred Stock (each of the foregoing, a “Mandatory Conversion Date”), all of the outstanding shares of Series A Convertible Preferred Stock will automatically convert to shares of our common stock (a “Mandatory Conversion”). In addition, each share of Series A Convertible Preferred Stock shall be convertible, at any time and from time to time at the option of the holder thereof, and without the payment of additional consideration by the holder thereof, into that number of shares of our common stock determined by dividing the Stated Value of such Series A Convertible Preferred Stock by the conversion price. The conversion price initially is $5.00 per share of common stock and is subject to adjustment described below.
Conversion Price Adjustment:
Stock Dividends and Stock Splits. If we pay a stock dividend or otherwise make a distribution payable in shares of our common stock on shares of our common stock or any other common stock equivalents, subdivide or combine outstanding common stock, or reclassify common stock, the conversion price will be adjusted by multiplying the then conversion price by a fraction, the numerator of which shall be the number of shares of our common stock outstanding immediately before such event, and the denominator of which shall be the number of shares outstanding immediately after such event.
Fundamental Transaction. If we effect a fundamental transaction, then upon any subsequent conversion of Series A Convertible Preferred Stock, the holder thereof shall have the right to receive, for each share of our common stock that would have been issuable upon such conversion immediately prior to the occurrence of such fundamental transaction, the number of shares of the successor’s or acquiring corporation’s common stock or of our common stock, if we are the surviving corporation, and any additional consideration receivable as a result of such fundamental transaction by a holder of the number of shares of our common stock into which Series A Convertible Preferred Stock is convertible immediately prior to such fundamental transaction. A fundamental transaction means: (i) our merger or consolidation with or into another entity, (ii) any sale of all or substantially all of our assets in one transaction or a series of related transactions, or (iii) any reclassification of our common stock or any compulsory share exchange by which our common stock is effectively converted into or exchanged for other securities, cash or property.
Voting Rights. Except as otherwise provided in the Certificate of Designations of Preferences, Rights and Limitations of Series A Convertible Preferred Stock (the “Certificate of Designations”) or required by law, Series A Convertible Preferred Stock shall have no class voting rights. The Certificate of Designations provides that each share of Series A Convertible Preferred Stock will entitle its holder to vote with the common stock on an as-if-converted to shares of our common stock basis. Notwithstanding certain protections in the Certificate of Designations, Delaware law also provides holders of preferred stock with certain rights. The holders of the outstanding shares of Series A Convertible Preferred Stock generally will be entitled to vote as a class upon a proposed amendment to our certificate of incorporation if the amendment would:
| 78 |
| ● | increase or decrease the aggregate number of authorized shares of our Series A Convertible Preferred Stock; | |
| ● | increase or decrease the par value of the shares of our Series A Convertible Preferred Stock; or | |
| ● | alter or change the powers, preferences, or special rights of the shares of our Series A Convertible Preferred Stock so as to affect them adversely. |
Fractional Shares. No fractional shares of our common stock will be issued upon conversion of Series A Convertible Preferred Stock. Rather, we shall round up to the next whole share.
Royalty Payment Rights:
Royalties. If and when the Company generates sales of the current and potential future versions of the Pure-Vu system, including disposables, parts, and services, or if the Company receives any proceeds from the licensing of the current and potential future versions of the Pure-Vu system, then Company will pay to the holders of our Series A Convertible Preferred Stock (the “Holders”) with the allocation of such Royalty Payment Rights between Holders determined as set forth below under “Allocation of Royalty Payments”, a royalty equal to, in the aggregate, in royalty payments in any calendar year for all products:
| The Company Commercializes Product Directly | The Rights to Commercialize the Product is Sublicensed by the Company to a third-party | |
| 3% of Net Sales* | 5% of any Licensing Proceeds** |
* Subject in all cases for all products to a maximum per calendar year equal to $30,000,000 (the total dollar amount of Units closed on in the 2017 Private Placement). Net Sales is defined in the Certificate of Designations.
** Subject in all cases for all products to a maximum per calendar year equal to $30,000,000 (the total dollar amount of Units closed on in the 2017 Private Placement). Licensing Proceeds is defined in the Certificate of Designations.
The Company currently has not licensed any of its products to any third-party and is not in negotiations with respect to any such license. There is no guarantee that the Company will ever generate sales of, or Licensing Proceeds from, its products. The Holders may never receive any royalty payments and these Royalty Payment Rights may expire worthless.
Timing of Royalty Payments. With respect to Pure-Vu system products that the Company commercializes directly, royalty payments, if any, will be paid on an annual basis 15 business days after the issuance of the Company’s audited financial statements for the prior year. With respect to Pure-Vu system products that the Company sublicenses to a third-party, royalty payments, if any, will be paid 10 business days after the end of the applicable quarter in which such Licensing Proceeds are received by the Company. However, all royalty payments shall be accrued by the Company until 15 business days after the issuance of the Company’s audited financial statements for the earlier of (i) the calendar year in which Net Sales exceed $15,000,000 or Licensing Proceeds exceed $2,500,000, or (ii) the year ended December 31, 2019, at which time all accrued royalties shall be paid in a lump sum along with the regular royalty payments and subsequent royalty payments will be made irrespective of the amount of annual Net Sales or Licensing Proceeds.
The royalty will be payable up to the later of (i) the latest expiration date for the Company’s current patents (which is currently October 2026), or (ii) the latest expiration date of any pending patents as of the date of the Initial Closing that may be issued in the future. Following the expiration of all such patents, the Holders of the Royalty Payment Rights will no longer be entitled to any further royalties for any period following the latest to occur of such patent expiration.
| 79 |
Royalty Vesting. The Royalty Payment Rights associated with the shares of Series A Convertible Preferred Stock will be immediately vested upon issuance of the shares.
If a Holder elects to convert all of his Series A Convertible Preferred Stock into shares of our common stock prior to December 22, 2019, the Holder will forfeit any and all rights to future Royalty Payments, if any. If a Holder elects to convert any portion of his Series A Convertible Preferred Stock to common stock at any time prior to December 22, 2019, such Holder will forfeit any rights to future Royalty Payments, if any, with respect to such converted shares.
Prior to December 22, 2019, the right to receive a royalty will follow the Series A Convertible Preferred Stock. In the event that an investor transfers any of its Series A Convertible Preferred Stock prior to December 22, 2019, the transferee of such shares will thereafter have the right to receive any and all royalty payments related to the Series A Convertible Preferred Stock it received, including with respect to royalty rights, and the transferring investor will thereafter no longer have any right to receive any royalty payment in respect of the Series A Convertible Preferred Stock it transferred.
Allocation of Royalty Payment. Once the aggregate Royalty Payment Amount is calculated based on the criteria set forth above under “Royalties,” that amount will be allocated to the holders of the Participating Royalty Interests (as defined in the Certificate of Designations) based on their pro rata ownership. An investor’s initial pro-rata ownership will be the investor’s number of Series A Convertible Preferred Stock as a percentage of the total number of such Shares issued in the 2017 Private Placement. The royalty payable to each holder shall be calculated as follows:
(i) Prior to December 22, 2019, the royalty payable to each holder will be equal to the aggregate Royalty Payment Amount divided by the aggregate Participating Royalty Interests on the applicable record date multiplied by the number of Participating Royalty Interests held by such holder the applicable record date.
(ii) On or after December 22, 2019, the Royalty payable to each holder will be calculated by multiplying the aggregate Royalty Payment Amount by the percentage set forth in each holder’s Royalty Payment Rights certificate. The percentage set forth in each Royalty Payment Rights certificate will be calculated as follows:
| Number of Participating Royalty Interests Held by Investor after December 22, 2019 |
| Total Participating Royalty Interests after December 22, 2019 |
Separability. The Royalty Payment Rights may not be transferred separately from the Series A Convertible Preferred Stock until after December 22, 2019. Prior to December 22, 2019, if a Holder transfers any of its Series A Convertible Preferred Stock, such Holder will lose any and all rights to any future royalty payments with respect to Series A Convertible Preferred Stock that were transferred. Following December 22, 2019, the Company will issue a certificate representing the Royalty Payment Rights to each Holder of Series A Convertible Preferred Stock at such date (the “Royalty Rights Certificate”). Following the issuance of the Royalty Rights Certificate, such Royalty Payment Rights may be transferred, subject to the availability of an exemption from registration under applicable state and federal securities laws.
Unsecured Obligations. The Royalty Payment Rights are unsecured obligations of the Company.
Warrants
Exchange Warrants. In connection with the Share Exchange Transaction and the CNA, we issued warrants to each former Convertible Holder to purchase an aggregate 907,237 shares of our common stock (the “Exchange Warrants”). The Exchange Warrants are exercisable for our common stock at an exercise price equal to $5.00 per share (the “Exercise Price”). The Exchange Warrants are exercisable immediately upon issuance and have a five year term, and provide for cashless exercise. The Exchange Warrants may be exercised at any time in whole or in part at the applicable exercise price until expiration of the Exchange Warrants. No fractional shares will be issued upon the exercise of the Exchange Warrants.
| 80 |
Placement Agent Warrants. In connection with completion of the 2017 Private Placement, we issued Aegis Capital (the “Placement Agent”), and its designees, warrants to purchase 403,632 shares of our common stock at an exercise price of $5.00 as partial compensation (the “Placement Agent Warrants”). These warrants have a five year term and provide cashless exercise.
Service Provider Warrants. As partial compensation, we issued a service provider warrants to purchase 30,000 shares of our common stock at an exercise price of $8.00. These warrants have a five year term and do not provide for cashless exercise.
Placement Agent Royalty Payment Rights
In connection with completion of the 2017 Private Placement, we issued the Placement Agent royalty payment rights certificates (the “Placement Agent Royalty Payment Rights Certificates”) to receive, in the aggregate, 10% of the amount of payments paid to the holders of the Series A Convertible Preferred Stock. The Placement Agent Royalty Payment Rights Certificates are on substantially similar terms as the Royalty Payment Rights of the Series A Convertible Preferred Stock.
Registration Rights
In connection with the 2017 Private Placement, we entered into a registration rights agreement (the “Registration Rights Agreement”) with the 2017 Private Placement investors, (the “Investors”). We were required to file with the SEC after the date of the final closing of the 2017 Private Placement (the “Registration Filing Date”), a registration statement (the “Resale Registration Statement”) covering the resale of the shares of our common stock held by the Investors (the “Investor Shares”) issued in the 2017 Private Placement, as well as the shares of our common stock underlying the Series A Convertible Preferred Stock issued in the 2017 Private Placement (together with the Investor Shares, the “Registrable Securities”). We are also required to use commercially reasonable efforts to have the Resale Registration Statement declared effective within one hundred and fifty (150) days after the Resale Registration Statement is filed (the “Effectiveness Deadline”); provided however, that if the Company signs a letter of intent or comparable agreement with an underwriter which contemplates an Initial Public Offering (“IPO”) or holds an organizational meeting for an IPO, or otherwise orally engages an underwriter to begin working with the Company towards an IPO prior to the Effectiveness Deadline (the “IPO Process Commencement Date”), then the Company shall file a joint registration statement covering the primary shares to be issued in the IPO and the resale of the Registrable Securities, and in such event the Registration Filing Date shall be extended to a date that is seventy five (75) calendar days after the IPO Process Commencement Date and the Effectiveness Deadline shall be extended to a date that is one hundred twenty (120) calendar days after the initial filing of the Registration Statement with the SEC. If the IPO is abandoned at any time, then the Registration Filing Date will be sixty (60) calendar days from the actual date of abandonment and the Effectiveness Deadline will be one hundred and fifty (150) calendar days after the date of abandonment. We are also required to keep the Resale Registration Statement continuously effective under the Securities Act for a period of one year or for such shorter period ending on the earlier to occur of the date when all the Registrable Securities covered by the Resale Registration Statement have been sold or such time as all of the Registrable Securities covered by the Resale Registration Statement can be sold under Rule 144 without any volume limitations.
If this Resale Registration Statement is not declared effective on or before the Effectiveness Deadline, we will be required to pay to each holder of Registrable Securities purchased in the 2017 Private Placement an amount in cash equal to one-half of one percent (0.5%) of such holder’s investment amount on every thirty (30) day anniversary of such Effectiveness Deadline until such failure is cured. The payment amount shall be prorated for partial thirty (30) day periods. The maximum amount of payments to be made by us to each Investor as the result of such failure, shall be an amount equal to six percent (6%) of each Investor’s investment amount. Notwithstanding the foregoing, no payments shall be owed with respect to any period during which all of the holder’s Registrable Securities may be sold by such holder without restriction under Rule 144. The Effectiveness Deadline for the Resale Registration Statement was September 9, 2017.
| 81 |
If declared effective, we are also required to keep the Resale Registration Statement “evergreen” for one (1) year from the date it is declared effective by the SEC or until Rule 144 of the Securities Act is available to the holders of Registrable Securities purchased in the 2017 Private Placement with respect to all of their shares, whichever is earlier.
We must pay all costs and expenses incurred by us in complying with our obligations to file the Resale Registration Statement pursuant to the Registration Rights Agreement, except that the selling holders will be responsible for their shares of the attorney’s fees and expenses and any commissions or other compensation to selling agents and similar persons; provided, however, that, in any registration, each party will pay for its own underwriting discounts and commissions and transfer taxes.
Lock-Up Agreements
Each of our directors and officers and the holders of substantially all of five percent (5%) or more of our common stock have agreed that they will not (a) offer, sell, contract to sell, grant any option to purchase, hypothecate, pledge or otherwise dispose of or (b) transfer title to any shares of our common stock acquired prior to the 2017 Private Placement, which includes any shares of our common stock acquired upon the exercise of any warrants acquired prior to the 2017 Private Placement, for a period beginning on December 22, 2016 and ending twelve (12) months following the effective date of the registration statement of which this prospectus is a part, without our prior written consent and the prior written consent of the Placement Agent.
In connection with the formation of Motus GI Holdings, Inc. (“Holdings”) in September, 2015, certain affiliates of the Placement Agent and certain other parties not affiliated with us or the Placement Agent subscribed for an aggregate of 1,650,000 shares of our common stock (the “Formation Shares”), for which they paid an aggregate of $82,000 ($0.05 per share). Each of the holders of the Formation Shares have agreed that they will not (a) offer, sell, contract to sell, grant any option to purchase, hypothecate, pledge or otherwise dispose of or (b) transfer title to any shares of our common stock acquired prior to the 2017 Private Placement, which includes any shares of our common stock acquired upon the exercise of any warrants acquired prior to the 2017 Private Placement, for a period beginning on December 22, 2016 and ending twelve (12) months following the effective date of the registration statement of which this prospectus is a part, without our prior written consent and the prior written consent of the Placement Agent.
In March 2017, the Company signed a consulting service agreement by which it granted the service provider 90,000 shares of the Company’s common stock (the “Consultant Shares”). The consultant has agreed that they will not (a) offer, pledge, sell, contract to sell, grant any option or contract to purchase, purchase any option or contract to sell, or otherwise dispose of, directly or indirectly, or (b) transfer title to any of the subject shares, for a period beginning the effective date of the consulting agreement and ending: (i) with respect to 22,500 of the Consultant Shares, upon the nine (9) month anniversary of the signing of the consulting agreement, (ii) with respect to 22,500 of the Consultant Shares, upon the six (6) month anniversary from the effective date of the registration statement of which this prospectus is a part, and (iii) with respect to 45,000 of the Consultant Shares, upon the twelve month anniversary from the effective date of the registration statement of which this prospectus is a part, without the prior written consent of the Company.
Transfer Agent and Registrar
Continental Stock Transfer and Trust, located at 1 State Street 30th Floor, New York, NY 10004, is the transfer agent and registrar for our common stock and preferred stock.
Quotation of Securities
Shares of our common stock will be listed on the NASDAQ Capital Market under the trading symbol “MOTS”.
| 82 |
Anti-Takeover Effect of Delaware Law, Certain Charter and Bylaw Provisions
Our certificate of incorporation and bylaws contain provisions that could have the effect of discouraging potential acquisition proposals or tender offers or delaying or preventing a change of control of our company. These provisions are as follows:
| ● | they provide that special meetings of stockholders may be called by the board of directors or at the request in writing by stockholders of record owning at least twenty (20%) percent of the issued and outstanding voting shares of our common stock; | |
| ● | they do not include a provision for cumulative voting in the election of directors. Under cumulative voting, a minority stockholder holding a sufficient number of shares may be able to ensure the election of one or more directors. The absence of cumulative voting may have the effect of limiting the ability of minority stockholders to effect changes in our board of directors; and | |
| ● | they allow us to issue, without stockholder approval, up to 10,000,000 shares of preferred stock that could adversely affect the rights and powers of the holders of our common stock. |
We are subject to the provisions of Section 203 of the General Corporation Law of the State of Delaware, an anti-takeover law. In general, Section 203 prohibits a publicly held Delaware corporation from engaging in a “business combination” with an “interested stockholder” for a period of three years after the date of the transaction in which the person became an interested stockholder, unless the business combination is approved in the following prescribed manner:
| ● | prior to the time of the transaction, the board of directors of the corporation approved either the business combination or the transaction which resulted in the stockholder becoming an interested stockholder; | |
| ● | upon completion of the transaction that resulted in the stockholder becoming an interested stockholder, the stockholder owned at least eighty-five percent (85%) of the voting stock of the corporation outstanding at the time the transaction commenced, excluding for purposes of determining the number of shares outstanding; (1) shares owned by persons who are directors and also officers and (2) shares owned by employee stock plans in which employee participants do not have the right to determine confidentially whether shares held subject to the plan will be tendered in a tender or exchange offer; and | |
| ● | on or subsequent to the time of the transaction, the business combination is approved by the board and authorized at an annual or special meeting of stockholders, and not by written consent, by the affirmative vote of at least sixty-six and two-thirds percent (66 2/3%) of the outstanding voting stock which is not owned by the interested stockholder. |
Generally, for purposes of Section 203, a “business combination” includes a merger, asset or stock sale, or other transaction resulting in a financial benefit to the interested stockholder. An “interested stockholder” is a person who, together with affiliates and associates, owns or, within three (3) years prior to the determination of interested stockholder status, owned fifteen percent (15%) or more of a corporation’s outstanding voting securities.
Choice of Forum
Our certificate of incorporation, as amended, provides that, unless we consent in writing to the selection of an alternative forum, the Court of Chancery of the State of Delaware will be the exclusive forum for any derivative action or proceeding brought on our behalf; any action asserting a breach of fiduciary duty; any action asserting a claim against us, or any of our officers or Directors, arising pursuant to the Delaware General Corporation Law, our certificate of incorporation, as amended, or our bylaws; or any action asserting a claim against us that is governed by the internal affairs doctrine. This exclusive forum provision may limit the ability of our stockholders to bring a claim in a judicial forum that such stockholders find favorable for the disputes listed above, which may discourage such lawsuits against us, or any of our officers or directors.
| 83 |
SHARES ELIGIBLE FOR FUTURE SALE
Prior to this offering, there has been no market for our common stock, and a liquid trading market for our common stock may not develop or be sustained after this offering. Future sales of substantial amounts of our common stock in the public market, or the perception that such sales could occur, could adversely affect market prices prevailing from time to time and could impair our ability to raise capital through the sale of our equity securities. Furthermore, because only a limited number of shares will be available for sale shortly after this offering due to existing contractual and legal restrictions on resale as described below, there may be sales of substantial amounts of our common stock in the public market after the restrictions lapse. This may adversely affect the prevailing market price and our ability to raise equity capital in the future. Although we have applied to have our common stock approved for listing on the NASDAQ Capital Market under the symbol “MOTS,” we cannot assure you that there will be an active public market for our common stock.
Based on the number of shares outstanding as of September 30, 2017, upon completion of this offering, shares of common stock will be outstanding. Of the shares to be outstanding immediately after the completion of this offering, the shares of common stock to be sold in this offering will be freely tradable without restriction under the Securities Act unless purchased by our “affiliates,” as that term is defined in Rule 144 under the Securities Act. In addition, any shares sold in this offering to entities affiliated with our existing stockholders, including stockholders affiliated with certain of our directors, will be subject to lock-up agreements.
The remaining 10,491,841 shares of common stock will be “restricted securities” under Rule 144.
Subject to the lock-up agreements described below and the provisions of Rule 144 and 701 under the Securities Act, these restricted securities will be available for sale in the public market as follows:
Date Available for Sale |
Shares Eligible for Sale |
Description | ||
| Date of Prospectus | Shares sold in the offering that are not subject to a lock-up | |||
| 90 Days after Date of Prospectus | Shares saleable under Rules 144 and 701 that are not subject to a lock-up | |||
| 180 Days after Date of Prospectus | Lock-up released; shares saleable under Rules 144 and 701 | |||
In addition:
| ● | of the 1,836,844 shares of our common stock that were issuable upon the exercise of stock options outstanding as of September 30, 2017, options to purchase 506,453 shares of common stock were exercisable as of that date, and upon exercise these shares will be eligible for sale subject to the lock-up agreements described below and Rules 144 and 701 under the Securities Act; | |
| ● | all 1,340,869 shares of our common stock that are issuable upon the exercise of warrants outstanding as of September 30, 2017 are currently exerciseable, and upon exercise these shares will be eligible for sale subject to the lock-up agreements described below and Rules 144 and 701 under the Securities Act; and | |
| ● | all 1,581,128 shares of our common stock that are issuable upon the conversion of our Series A Convertible Preferred Stock outstanding as of September 30, 2017 are currently eligible for voluntary conversion, and upon such voluntary conversion these shares will be eligible for sale subject to the lock-up agreements described below and Rules 144 and 701 under the Securities Act. |
| 84 |
Rule 144
In general, under Rule 144 as currently in effect, once we have been subject to the reporting requirements under the Exchange Act for at least 90 days, a person (or persons whose shares are aggregated) who is not deemed to have been an affiliate of ours at any time during the three months preceding a sale, and who has beneficially owned restricted securities within the meaning of Rule 144 for at least six months, would be entitled to sell those shares, subject only to the availability of current public information about us. A non-affiliated person who has beneficially owned restricted securities within the meaning of Rule 144 for at least one year would be entitled to sell those shares without regard to the provisions of Rule 144.
An affiliate of ours who has beneficially owned restricted shares of our common stock for at least one year (or six months, provided that such sale occurs after we have been subject to the reporting requirements under the Exchange Act for at least 90 days) would be entitled to sell, within any three-month period, a number of shares that does not exceed the greater of:
| ● | 1% of shares of our common stock then outstanding; or | |
| ● | the average weekly trading volume of shares of our common stock on the NASDAQ Capital Market during the four calendar weeks preceding the date on which notice of the sale is filed with the SEC. |
Sales under Rule 144 by our affiliates or persons selling shares on behalf of our affiliates are also subject to manner of sale provisions, notice requirements and the availability of current public information about us.
Rule 701
In general, under Rule 701 as currently in effect, any of our employees, directors, officers, consultants or advisors who acquired common stock from us in connection with a written compensatory stock or option plan or other written agreement in compliance with Rule 701 under the Securities Act before the effective date of a registration statement is entitled to rely on Rule 701 to resell such shares in reliance on Rule 144. An affiliate of the issuer can resell shares in reliance on Rule 144 without having to comply with the holding period requirements of Rule 144, and a non-affiliate of the issuer can resell shares in reliance on Rule 144 without having to comply with the holding period requirements of Rule 144 and without regard to the volume of such sales or the availability of public information about the issuer. However, substantially all Rule 701 shares are subject to lock-up agreements as described below and under the section entitled “Underwriting” and will become eligible for sale upon the expiration of the restrictions set forth in those agreements.
Lock-up Agreements
Each of our directors and officers and the holders of substantially all of five percent (5%) or more of our common stock have agreed that they will not (a) offer, sell, contract to sell, grant any option to purchase, hypothecate, pledge or otherwise dispose of or (b) transfer title to any shares of our common stock acquired prior to the 2017 Private Placement, which includes any shares of our common stock acquired upon the exercise of any warrants acquired prior to the 2017 Private Placement, for a period beginning on December 22, 2016 and ending twelve (12) months following the effective date of the registration statement of which this prospectus is a part, without our prior written consent and the prior written consent of the Placement Agent.
In connection with the formation of Motus GI Holdings, Inc. (“Holdings”) in September, 2015, certain affiliates of the Placement Agent and certain other parties not affiliated with us or the Placement Agent subscribed for an aggregate of 1,650,000 shares of our common stock (the “Formation Shares”), for which they paid an aggregate of $82,000 ($0.05 per share). Each of the holders of the Formation Shares have agreed that they will not (a) offer, sell, contract to sell, grant any option to purchase, hypothecate, pledge or otherwise dispose of or (b) transfer title to any shares of our common stock acquired prior to the 2017 Private Placement, which includes any shares of our common stock acquired upon the exercise of any warrants acquired prior to the 2017 Private Placement, for a period beginning on December 22, 2016 and ending twelve (12) months following the effective date of the registration statement of which this prospectus is a part, without our prior written consent and the prior written consent of the Placement Agent.
| 85 |
In March 2017, the Company signed a consulting service agreement by which it granted the service provider 90,000 shares of the Company’s common stock (the “Consultant Shares”). The consultant has agreed that they will not (a) offer, pledge, sell, contract to sell, grant any option or contract to purchase, purchase any option or contract to sell, or otherwise dispose of, directly or indirectly, or (b) transfer title to any of the subject shares, for a period beginning the effective date of the consulting agreement and ending: (i) with respect to 22,500 of the Consultant Shares, upon the nine (9) month anniversary of the signing of the consulting agreement, (ii) with respect to 22,500 of the Consultant Shares, upon the six (6) month anniversary from the effective date of the registration statement of which this prospectus is a part, and (iii) with respect to 45,000 of the Consultant Shares, upon the twelve month anniversary from the effective date of the registration statement of which this prospectus is a part, without the prior written consent of the Company.
Our officers and directors and the holders of substantially all of our outstanding shares of capital stock have agreed with the underwriters, subject to certain exceptions, not to dispose of or hedge any of their common stock or securities convertible into or exchangeable for shares of common stock for a period through the date 180 days after the date of this prospectus, except with the prior written consent of , as the representative of the underwriters. The representative may in their sole discretion and at any time without notice release some or all of the shares subject to lock-up agreements prior to the expiration of the 180-day period. When determining whether or not to release shares from the lock-up agreements, the representative may consider, among other factors, the stockholder’s reasons for requesting the release, the number of shares for which the release is being requested and market conditions at the time.
Registration Rights
Certain holders of our securities are entitled to various rights with respect to registration of their shares under the Securities Act. Registration of these shares under the under the Securities Act would result in these shares becoming fully tradeable without restriction under the Securities Act immediately upon the effectiveness of the registration. See the section entitled “Description of Securities – Registration Rights” for additional information.
Equity Incentive Plans
We intend to file one or more registration statements on Form S-8 under the Securities Act to register our shares issued or reserved for issuance under our equity incentive plans. The first such registration statement is expected to be filed soon after the date of this prospectus and will automatically become effective upon filing with the SEC. Accordingly, shares registered under such registration statement will be available for sale in the open market, unless such shares are subject to vesting restrictions with us or the lock-up restrictions described above. As of September 30, 2017, we estimate that such registration statement on Form S-8 will cover approximately 2,011,656 shares.
| 86 |
MATERIAL U.S. FEDERAL INCOME TAX CONSEQUENCES TO NON-U.S. HOLDERS OF OUR COMMON STOCK
The following is a summary of the material United States federal income tax consequences to non-U.S. holders (as defined below) of their ownership and disposition of our common stock, but does not purport to be a complete analysis of all the potential tax considerations relating thereto. This summary is based upon current provisions of the Code, existing and proposed United States Treasury Regulations promulgated thereunder, current administrative rulings, and judicial decisions, all as in effect as of the date hereof. Especially in light of recent legislative proposals, these authorities may be changed, possibly retroactively, so as to result in United States federal tax consequences different from those set forth below. We have not obtained, and do not intend to obtain, any opinion of counsel or ruling from the Internal Revenue Service, or the IRS, with respect to the statements made and the conclusions reached in the following summary, and there can be no assurance that the IRS will agree with such statements and conclusions.
This summary also does not address the tax considerations arising under the laws of any non-United States, state or local jurisdiction or under any non-income tax laws, including United States federal gift and estate tax laws, except to the limited extent set forth below. In addition, this discussion does not address the potential application of the tax on net investment income or the alternative minimum tax. This discussion may not apply, in whole or in part, to particular non-U.S. holders in light of their individual circumstances or to holders subject to special treatment under the United States federal income tax laws, including, without limitation:
| ● | insurance companies, banks or other financial institutions; | |
| ● | tax-exempt organizations; | |
| ● | pension plans; | |
| ● | controlled foreign corporations or passive foreign investment companies; | |
| ● | brokers or dealers in securities or currencies; | |
| ● | traders in securities that elect to use a mark-to-market method of accounting for their securities holdings; | |
| ● | persons that own, or are deemed to own, more than 5% of our capital stock (except to the extent specifically set forth below); | |
| ● | certain former citizens or long-term residents of the United States; | |
| ● | persons that hold our common stock as a position in a hedging transaction, straddle, conversion transaction, synthetic security or other integrated investment; | |
| ● | persons that hold or receive our common stock pursuant to the exercise of any employee stock option or otherwise as compensation; and | |
| ● | persons that do not hold our common stock as a capital asset within the meaning of Section 1221 of the Code. |
In addition, this discussion does not address the tax treatment of partnerships, including any entity or arrangement treated as a partnership for United States federal income tax purposes. Generally, the tax treatment of a person treated as a partner in such an entity will depend on the status of the partner, the activities of the partner and the partnership, and certain determinations made at the partner level. Accordingly, partnerships that hold our common stock, and partners in such partnerships, should consult their tax advisors.
THIS SUMMARY IS NOT INTENDED TO BE CONSTRUED AS LEGAL ADVICE. WE RECOMMEND THAT PROSPECTIVE INVESTORS CONSULT THEIR TAX ADVISORS REGARDING THE PARTICULAR U.S. FEDERAL INCOME TAX CONSEQUENCES TO THEM OF THE ACQUISITION, OWNERSHIP AND DISPOSITION OF OUR COMMON STOCK, AS WELL AS ANY TAX CONSEQUENCES ARISING UNDER ANY STATE, LOCAL OR FOREIGN TAX LAWS, ANY APPLICABLE INCOME TAX TREATIES, OR ANY OTHER U.S. FEDERAL TAX LAWS (INCLUDING ESTATE AND GIFT TAX LAWS).
| 87 |
Definition of Non-U.S. Holder
For purposes of this discussion, you are a non-U.S. holder if you are a beneficial owner of our common stock that is not, for United States federal income tax purposes:
| ● | an individual citizen or resident of the United States; | |
| ● | a corporation, or other entity taxable as a corporation, created or organized in the United States or under the laws of the United States or any political subdivision thereof; | |
| ● | an estate whose income is subject to United States federal income tax regardless of its source; or | |
| ● | a trust whose administration is subject to the primary supervision of a United States court and which has one or more “United States persons” (as defined in the Code) who have the authority to control all substantial decisions of the trust, or which has made a valid election to be treated as a United States person. |
Distributions to non-U.S. Holders
As described in the section titled “Dividend Policy,” we do not anticipate paying any cash dividends or making distributions of other property on our common stock in the foreseeable future. However, if we do make distributions of cash or property on our common stock, those payments will constitute dividends for United States tax purposes to the extent paid from our current or accumulated earnings and profits, as determined under United States federal income tax principles. To the extent those distributions exceed both our current and our accumulated earnings and profits, the excess will constitute a return of capital and will first reduce a non-U.S. holder’s tax basis in our common stock, but not below zero, and then will be treated by a non-U.S. holder as gain from the sale of stock as described below under “Gain on dispositions of our common stock by non-U.S. holders.”
Subject to the discussion below on effectively connected income, any dividend paid to a non-U.S. holder generally will be subject to United States withholding tax either at a rate of 30% of the gross amount of the dividend or such lower rate as may be specified by an applicable income tax treaty. To receive a reduced treaty rate, a non-U.S. holder must provide us with an IRS Form W-8BEN or W-8BEN-E (or applicable successor form) and certify qualification for the reduced rate. If a non-U.S. holder is eligible for a reduced rate of United States withholding tax pursuant to an income tax treaty, such non-U.S. holder may obtain a refund or credit of any excess amounts withheld by timely filing an appropriate claim with the IRS. If a non-U.S. holder holds our common stock through a financial institution or other agent acting on such non-U.S. holder’s behalf, appropriate documentation will need to be provided to the agent, which then will be required to provide certification to us or our paying agent, either directly or through other intermediaries.
Dividends received by a non-U.S. holder that are effectively connected with such non-U.S. holder’s conduct of a trade or business in the United States (and, if an applicable income tax treaty so provides, that are attributable to a permanent establishment or a fixed base maintained by such non-U.S. holder in the United States), are generally exempt from the 30% withholding tax if certain certification and disclosure requirements are satisfied. To obtain this exemption, a non-U.S. holder must provide us with an IRS Form W-8ECI (or applicable successor form) properly certifying such exemption. However, such effectively connected dividends, although not subject to withholding tax, generally are taxed at the same graduated United States federal income tax rates applicable to United States persons, net of certain deductions and credits. In addition, dividends received by a corporate non-U.S. holder that are effectively connected with the conduct of a trade or business in the United States may also be subject to a branch profits tax at a rate of 30% or such lower rate as may be specified by an applicable income tax treaty. Non-U.S. holders should consult with tax advisors regarding any applicable income tax or other treaties that may provide for different rules.
Any documentation provided to an applicable withholding agent may need to be updated in certain circumstances. The certification requirements described above also may require a non-U.S. holder to provide a United States taxpayer identification number.
For additional withholding rules that may apply to dividends, including dividends paid to foreign financial institutions (as specifically defined by the applicable rules) or to certain other foreign entities that have substantial direct or indirect United States owners, see the discussion below under the headings “Backup withholding and information reporting” and “Withholdable payments to foreign financial institutions and other foreign entities.”
| 88 |
Gain on Disposition of Our Common Stock by non-U.S. Holders
Subject to the discussion below under the headings “Backup withholding and information reporting” and “Withholdable payments to foreign financial institutions and other foreign entities,” a non-U.S. holder generally will not be required to pay United States federal income tax or withholding tax on any gain recognized upon the sale, exchange or other taxable disposition of our common stock unless:
| ● | the gain is effectively connected with the conduct of a trade or business by such non-U.S. holder in the United States (and, if an applicable income tax treaty so provides, the gain is attributable to a permanent establishment or a fixed base maintained by such non-U.S. holder in the United States), in which case the non-U.S. holder will be required to pay tax on the net gain derived from the sale or disposition at the graduated rates and in the manner applicable to United States persons, and an additional branch profits tax at a rate of 30% (or such lower rate as may be specified by an applicable income tax treaty) may also apply to a corporate non-U.S. holder; | |
| ● | such non-U.S. holder is a nonresident alien individual who is present in the United States for a period or periods aggregating 183 days or more during the calendar year in which the sale or disposition occurs and certain other conditions are met, in which case the non-U.S. holder will be required to pay a flat 30% tax (or such lower rate as may be specified by an applicable income tax treaty) on the gain derived from the sale or disposition, which gain may be offset by United States-source capital losses for the taxable year of the sale or disposition; or | |
| ● | our common stock constitutes a United States real property interest by reason of our status as a “United States real property holding corporation”, or USRPHC, for United States federal income tax purposes at any time within the shorter of the five-year period preceding such non-U.S. holder’s disposition of, or holding period for, our common stock, in which case the non-U.S. holder generally will be taxed on net gain derived from the sale or disposition at the graduated rates applicable to United States persons. |
We believe that we are not currently and will not become a USRPHC and the remainder of this discussion so assumes. However, because the determination of whether we are a USRPHC depends on the fair market value of our United States real property relative to the fair market value of our other business assets, there can be no assurance that we will not become a USRPHC in the future. Even if we become a USRPHC, however, as long as our common stock is regularly traded on an established securities market, such common stock will be treated as United States real property interests only if a non-U.S. holder actually or constructively holds more than 5% of such regularly traded common stock at any time during the shorter of the five-year period preceding such non-U.S. holder’s disposition of, or holding period for, our common stock. Non- U.S. holders should consult with tax advisors regarding any applicable income tax or other treaties that may provide for different rules.
Information Reporting and Backup Withholding
We (or the applicable paying agent) must report annually to the IRS the amount of dividends on our common stock paid to non-U.S. holders and the amount of tax withheld, if any. A similar report will be sent to each non-U.S. holder. Copies of this information reporting may also be made available under the provisions of a specific income tax treaty or agreement with the tax authorities in a non-U.S. holder’s country of residence.
Non-U.S. holders will generally be subject to backup withholding (at a current rate of 28%) for dividends on our common stock paid to such non-U.S. holders unless an exemption is established such as by, for example, properly certifying non-United States status on an IRS Form W-8BEN or W-8BEN-E (or applicable successor form). Notwithstanding the foregoing, backup withholding and information reporting may apply if either we or our paying agent has actual knowledge, or reason to know, that a holder of our common stock is a United States person.
Information reporting and backup withholding generally are not required with respect to the amount of any proceeds from the sale or other disposition of our common stock by a non-U.S. holder outside the United States through a foreign office of a foreign broker that does not have certain specified connections to the United States. However, if a non-U.S. holder sells or otherwise disposes of shares of common stock through a United States broker or the United States offices of a foreign broker, the broker will generally be required to report the amount of proceeds paid to such non-U.S. holder to the IRS and also to backup withhold on that amount unless the broker is provided appropriate certification of status as a non-United States person or an exemption is otherwise established. Information reporting will also apply if a non-U.S. holder sells shares of common stock through a foreign broker deriving more than a specified percentage of its income from United States sources or having certain other connections to the United States, unless such broker has documentary evidence in its records that such non-U.S. holder is a non-United States person and certain other conditions are met, or an exemption is otherwise established.
| 89 |
Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules from a payment may be refunded or credited against a non-U.S. holder’s United States federal income tax liability, if any, provided that an appropriate claim is timely filed with the IRS. Non-U.S. holders should consult with tax advisors regarding the application of the information reporting and backup withholding rules to investment in our common stock.
Withholdable Payments to Foreign Financial Institutions and other Foreign Entities
The Foreign Account Tax Compliance Act, or FATCA, imposes a United States federal withholding tax of 30% on certain payments to “foreign financial institutions” (as specifically defined under these rules) and certain other non-United States persons that fail to comply with certain information reporting and certification requirements pertaining to their direct and indirect United States securityholders and/or United States account holders. Such payments include dividends on and, on or after January 1, 2019, gross proceeds from the sale or other disposition of our common stock. Under certain circumstances, a non-U.S. holder may be eligible for refunds or credits of such taxes. An intergovernmental agreement between the United States and an applicable foreign country may modify the requirements described in this paragraph. Non-U.S. holders should consult with tax advisors regarding the possible implications of this legislation and any applicable intergovernmental agreements on investment in our common stock.
U.S. Federal Estate Tax
Our common stock beneficially owned by an individual who is not a citizen or resident of the United States (as defined for United States federal estate tax purposes) at the time of their death will generally be includable in the decedent’s gross estate for United States federal estate tax purposes and, therefore, may be subject to United States federal estate tax unless an applicable estate tax treaty or other treaty provides otherwise. Investors are urged to consult their own tax advisors regarding the United States federal estate tax consequences of the ownership or disposition of our common stock.
NON-U.S. HOLDERS ARE URGED TO CONSULT WITH TAX ADVISORS WITH RESPECT TO THE APPLICATION OF THE UNITED STATES FEDERAL INCOME TAX LAWS TO THEIR PARTICULAR SITUATION, AS WELL AS ANY TAX CONSEQUENCES OF THE PURCHASE, OWNERSHIP AND DISPOSITION OF OUR COMMON STOCK ARISING UNDER THE UNITED STATES FEDERAL ESTATE OR GIFT TAX RULES OR UNDER THE LAWS OF ANY STATE, LOCAL, NON-UNITED STATES OR OTHER TAXING JURISDICTION OR UNDER ANY APPLICABLE TAX TREATY, INCLUDING THE CONSEQUENCES OF ANY PROPOSED CHANGE IN APPLICABLE LAWS.
| 90 |
We and the underwriters for the offering named below have entered into an underwriting agreement with respect to the common stock being offered. Subject to the terms and conditions of the underwriting agreement, each underwriter has severally agreed to purchase from us, at the initial public offering price less the underwriting discounts and commissions set forth on the cover page of this prospectus, the number of shares of our common stock set forth opposite its name below. is the representative of the underwriters.
| Underwriter | Number of Shares | |||
| Total | ||||
The underwriting agreement provides that the obligations of the underwriters are subject to certain conditions precedent and that the underwriters have agreed, severally and not jointly, to purchase all of the shares sold under the underwriting agreement if any of these shares are purchased, other than those shares covered by the overallotment option described below. If an underwriter defaults, the underwriting agreement provides that the purchase commitments of the non-defaulting underwriters may be increased or the underwriting agreement may be terminated. The underwriters reserve the right to withdraw, cancel or modify offers to the public and to reject orders in whole or in part.
Overallotment Option to Purchase Additional Shares. We have granted to the underwriters an option to purchase up to additional shares of common stock at the public offering price, less the underwriting discount. This option is exercisable for a period of 30 days. To the extent that the underwriters exercise this option, the underwriters will purchase additional shares from us in approximately the same proportion as shown in the table above. If any additional shares of our common stock are purchased, the underwriters will offer the additional shares on the same terms as those on which the shares are being offered.
Discounts and Commissions. The following table shows the public offering price, underwriting discount and proceeds, before expenses to us. These amounts are shown assuming both no exercise and full exercise of the underwriters’ option to purchase additional shares.
We estimate that the total expenses of the offering, excluding underwriting discount, will be approximately $ and are payable by us.
| Total | ||||||||||||
| Per Share | Without Overallotment | With Overallotment | ||||||||||
| Public offering price | ||||||||||||
| Underwriting discount | ||||||||||||
| Proceeds, before expenses, to us | ||||||||||||
The underwriters propose to offer the shares of common stock to the public at the public offering price set forth on the cover of this prospectus. The underwriters may offer the shares of common stock to securities dealers at the public offering price less a concession not in excess of $ per share. If all of the shares are not sold at the public offering price, the underwriters may change the offering price and other selling terms.
| 91 |
Discretionary Accounts. The underwriters do not intend to confirm sales of the shares to any accounts over which they have discretionary authority.
Market Information. Prior to this offering, there has been no public market for shares of our common stock. The initial public offering price will be determined by negotiations between us and the representative of the underwriters. In addition to prevailing market conditions, the factors to be considered in these negotiations will include:
| ● | the history of, and prospects for, our company and the industry in which we compete; | |
| ● | our past and present financial information; | |
| ● | an assessment of our management; its past and present operations, and the prospects for, and timing of, our future revenues; | |
| ● | the present state of our development; and | |
| ● | the above factors in relation to market values and various valuation measures of other companies engaged in activities similar to ours. |
An active trading market for the shares may not develop. It is also possible that after the offering the shares will not trade in the public market at or above the initial public offering price.
We have applied for the quotation of our common stock on under the symbol “MOTS”.
Stabilization. In connection with this offering, the underwriters may engage in stabilizing transactions, overallotment transactions, syndicate covering transactions, penalty bids and purchases to cover positions created by short sales.
| ● | Stabilizing transactions permit bids to purchase shares of common stock so long as the stabilizing bids do not exceed a specified maximum, and are engaged in for the purpose of preventing or retarding a decline in the market price of the common stock while the offering is in progress. | |
| ● | Overallotment transactions involve sales by the underwriters of shares of common stock in excess of the number of shares the underwriters are obligated to purchase. This creates a syndicate short position which may be either a covered short position or a naked short position. In a covered short position, the number of shares over-allotted by the underwriters is not greater than the number of shares that they may purchase in the overallotment option. In a naked short position, the number of shares involved is greater than the number of shares in the overallotment option. The underwriters may close out any short position by exercising their overallotment option or purchasing shares in the open market. | |
| ● | Syndicate covering transactions involve purchases of common stock in the open market after the distribution has been completed in order to cover syndicate short positions. In determining the source of shares to close out the short position, the underwriters will consider, among other things, the price of shares available for purchase in the open market as compared with the price at which they may purchase shares through exercise of the overallotment option. If the underwriters sell more shares than could be covered by exercise of the overallotment option and, therefore, have a naked short position, the position can be closed out only by buying shares in the open market. A naked short position is more likely to be created if the underwriters are concerned that after pricing there could be downward pressure on the price of the shares in the open market that could adversely affect investors who purchase in the offering. | |
| ● | Penalty bids permit the representative to reclaim a selling concession from a syndicate member when the common stock originally sold by that syndicate member is purchased in stabilizing or syndicate covering transactions to cover syndicate short positions. |
| 92 |
These stabilizing transactions, syndicate covering transactions and penalty bids may have the effect of raising or maintaining the market price of our common stock or preventing or retarding a decline in the market price of our common stock. As a result, the price of our common stock in the open market may be higher than it would otherwise be in the absence of these transactions. Neither we nor the underwriters make any representation or prediction as to the effect that the transactions described above may have on the price of our common stock. These transactions may be effected on , in the over-the-counter market or otherwise and, if commenced, may be discontinued at any time.
Passive Market Making. In connection with this offering, the underwriters and selling group members, if any, may engage in passive market making transactions in our common stock on in accordance with Rule 103 of Regulation M under the Securities Exchange Act of 1934, as amended, during a period before the commencement of offers or sales of common stock and extending through the completion of the distribution. A passive market maker must display its bid at a price not in excess of the highest independent bid of that security. However, if all independent bids are lowered below the passive market maker’s bid, such bid must then be lowered when specified purchase limits are exceeded.
Lock-Up Agreements. Pursuant to certain ’‘lock-up’’ agreements, we and our executive officers, directors and holders of substantially all of our common stock and securities convertible into or exchangeable for our common stock, including , have agreed, subject to certain exceptions, not to offer, sell, assign, transfer, pledge, contract to sell, or otherwise dispose of or announce the intention to otherwise dispose of, or enter into any swap, hedge or similar agreement or arrangement that transfers, in whole or in part, the economic consequence of ownership of, directly or indirectly, or make any demand or request or exercise any right with respect to the registration of, or file with the SEC a registration statement under the Securities Act relating to, any common stock or securities convertible into or exchangeable or exercisable for any common stock without the prior written consent of for a period of 180 days after the date of the pricing of the offering.
This lock-up provision applies to common stock and to securities convertible into or exchangeable or exercisable for common stock. It also applies to common stock owned now or acquired later by the person executing the agreement or for which the person executing the agreement later acquires the power of disposition, including any shares acquired by our directors and officers in the directed share program described below. The exceptions permit us, among other things and subject to restrictions, to: (1) issue common stock or options pursuant to employee benefit plans, (2) issue common stock upon exercise of outstanding options or warrants, or (3) file registration statements on Form S-8. The exceptions permit parties to the ’‘lock-up’’ agreements, among other things and subject to restrictions, to: (a) make certain gifts, (b) if the party is a corporation, partnership, limited liability company or other business entity, make transfers to any shareholders, partners, members of, or owners of similar equity interests in, the party, or to an affiliate of the party, if such transfer is not for value, and (c) if the party is a corporation, partnership, limited liability company or other business entity, make transfers in connection with the sale or transfer of all of the party’s capital stock, partnership interests, membership interests or other similar equity interests, as the case may be, or all or substantially all of the party’s assets, in any such case not undertaken for the purpose of avoiding the restrictions imposed by the “lock-up” agreement. In addition, the lock-up provision will not restrict broker-dealers from engaging in market making and similar activities conducted in the ordinary course of their business.
in their sole discretion, may release our common stock and other securities subject to the lock-up agreements described above in whole or in part at any time. When determining whether or not to release our common stock and other securities from lock-up agreements, will consider, among other factors, the holder’s reasons for requesting the release, the number of shares for which the release is being requested and market conditions at the time of the request. In the event of such a release or waiver for one of our directors or officers, shall provide us with notice of the impending release or waiver at least three business days before the effective date of such release or waiver and we will announce the impending release or waiver by issuing a press release at least two business days before the effective date of the release or waiver.
Electronic Offer, Sale and Distribution of Shares. A prospectus in electronic format may be made available on the websites maintained by one or more of the underwriters or selling group members, if any, participating in this offering and one or more of the underwriters participating in this offering may distribute prospectuses electronically. The representative may agree to allocate a number of shares to underwriters and selling group members for sale to their online brokerage account holders. Internet distributions will be allocated by the underwriters and selling group members that will make Internet distributions on the same basis as other allocations. Other than the prospectus in electronic format, the information on these websites is not part of this prospectus or the registration statement of which this prospectus forms a part, has not been approved or endorsed by us or any underwriter in its capacity as underwriter, and should not be relied upon by investors.
Other Relationships. Certain of the underwriters and their affiliates may in the future provide various investment banking, commercial banking and other financial services for us and our affiliates for which they may in the future receive customary fees.
Notice to Prospective Investors
Other than in the United States, no action has been taken by us or the underwriters that would permit a public offering of the securities offered by this prospectus in any jurisdiction where action for that purpose is required. The securities offered by this prospectus may not be offered or sold, directly or indirectly, nor may this prospectus or any other offering material or advertisements in connection with the offer and sale of any such securities be distributed or published in any jurisdiction, except under circumstances that will result in compliance with the applicable rules and regulations of that jurisdiction. Persons into whose possession this prospectus comes are advised to inform themselves about and to observe any restrictions relating to the offering and the distribution of this prospectus. This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any securities offered by this prospectus in any jurisdiction in which such an offer or a solicitation is unlawful.
| 93 |
The validity of the securities offered in this prospectus is being passed upon for us by Lowenstein Sandler LLP, New York, New York. has acted as counsel for the underwriters in connection with certain legal matters related to this offering.
The consolidated balance sheets of Motus GI Holdings, Inc., and the related statements of operations, stockholders’ deficit, and cash flows for each of the years in the two-year period ended December 31, 2016, have been audited by Brightman Almagor Zohar & Co., an independent registered public accounting firm, as stated in their report which is included in this prospectus herein. Such financial statements have been included herein in reliance on the report of such firm given upon their authority as experts in accounting and auditing.
DISCLOSURE OF COMMISSION POSITION ON INDEMNIFICATION FOR SECURITIES ACT LIABILITIES
Our directors and officers are indemnified to the fullest extent permitted under Delaware law. We also maintain insurance which protects our officers and directors against any liabilities incurred in connection with their service in such a capacity.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to our directors, officers and controlling persons pursuant to the foregoing, or otherwise, we have been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by us of expenses incurred or paid by a director, officer or controlling person of ours in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, we will, unless in the opinion of our counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
WHERE YOU CAN FIND ADDITIONAL INFORMATION
We have filed with the SEC a registration statement on Form S-1 under the Securities Act with respect to the common stock offered by this prospectus. This prospectus, which is part of the registration statement, omits certain information, exhibits, schedules and undertakings set forth in the registration statement. For further information pertaining to us and our common stock, reference is made to the registration statement and the exhibits and schedules to the registration statement. Statements contained in this prospectus as to the contents or provisions of any documents referred to in this prospectus are not necessarily complete, and in each instance where a copy of the document has been filed as an exhibit to the registration statement, reference is made to the exhibit for a more complete description of the matters involved.
You may read and copy all or any portion of the registration statement without charge at the office of the SEC at the Public Reference Room at Station Place, 100 F Street, N.E., Washington, D.C. 20549. Copies of the registration statement may be obtained from the SEC at prescribed rates from the Public Reference Section of the SEC at such address. In addition, registration statements and certain other filings made with the SEC electronically are publicly available through the SEC’s web site at http://www.sec.gov. The registration statement, including all exhibits and amendments to the registration statement, has been filed electronically with the SEC.
Upon completion of this offering, we will become subject to the information and periodic reporting requirements of the Securities Exchange Act of 1934, as amended, and, in accordance with such requirements, will file periodic reports, proxy statements, and other information with the SEC. These periodic reports, proxy statements, and other information will be available for inspection and copying at the regional offices, public reference facilities, and web site of the SEC referred to above. We intend to furnish our stockholders with annual reports containing consolidated financial statements audited by our independent registered accounting firm.
| 94 |

MOTUS GI HOLDINGS, INC.
CONSOLIDATED FINANCIAL STATEMENTS
MOTUS GI HOLDINGS, INC.
INDEX TO FINANCIAL STATEMENTS
Contents
| F-1 |
MOTUS GI HOLDINGS, INC.
INTERIM CONDENSED CONSOLIDATED BALANCE SHEETS
Expressed in U.S. dollars in thousands, except share data
| As of | As of | |||||||||||
| June 30, 2017 | December 31, 2016 | |||||||||||
| Note | Unaudited | |||||||||||
| ASSETS | ||||||||||||
| Current assets | ||||||||||||
| Cash and cash equivalents | 12,940 | 11,644 | ||||||||||
| Restricted cash | - | 7 | ||||||||||
| Accounts receivables | 18 | - | ||||||||||
| Inventory | 419 | 81 | ||||||||||
| Other current assets | 673 | 263 | ||||||||||
| Total current assets | 14,050 | 11,995 | ||||||||||
| Fixed assets, net | 589 | 141 | ||||||||||
| Long-term deposits | 106 | 55 | ||||||||||
| Total Assets | 14,745 | 12,191 | ||||||||||
| LIABILITIES AND SHAREHOLDERS’ EQUITY | ||||||||||||
| Current liabilities | ||||||||||||
| Trade accounts payable | 913 | 107 | ||||||||||
| Other current liabilities | 1,449 | 645 | ||||||||||
| Total current liabilities | 2,362 | 752 | ||||||||||
| Other long-term liabilities | 5 | 1,544 | 1,410 | |||||||||
| Shareholders’ equity (deficit) | 3 | |||||||||||
| Common stock - $0.0001 par value | ||||||||||||
| Authorized: 50,000,000 as of June 30, 2017 and December 31, 2016, respectively | 1 | 1 | ||||||||||
| Issued and outstanding: 10,489,066 and 9,294,463 as of June 30, 2017 and December 31, 2016, respectively | ||||||||||||
| Preferred series A stock - $0.0001 par value | (* | ) | (* | ) | ||||||||
| Authorized: 2,000,000 as of June 30, 2017 and December 31 2016, respectively. | ||||||||||||
| Issued and outstanding: 1,581,128 and 1,214,845 as of June 30, 2017 and December 31 2016 respectively. | ||||||||||||
| Preferred stock - $0.0001 par value | ||||||||||||
Authorized: 8,000,000 as of June 30, 2017 Issued and outstanding: 0 as of December 31, 2016 | - | - | ||||||||||
| Additional paid-in capital | 43,020 | 35,949 | ||||||||||
| Accumulated deficit | (32,182 | ) | (25,921 | ) | ||||||||
| Total shareholders’ equity | 10,839 | 10,029 | ||||||||||
| Total liabilities and shareholders’ equity | 14,745 | 12,191 | ||||||||||
| (*) | Represents an amount less than one thousand. |
The accompanying notes are an integral part of the interim condensed consolidated financial statements.
| F-2 |
MOTUS GI HOLDINGS, INC.
INTERIM CONDENSED CONSOLIDATED STATEMENTS OF COMPREHENSIVE LOSS
Expressed in U.S. dollars in thousands, except share and per share information
| Six months ended June 30, | Three months ended June 30, | ||||||||||||||||||
| 2017 | 2016 | 2017 | 2016 | ||||||||||||||||
| Note | Unaudited | Unaudited | |||||||||||||||||
| Revenue | 2B | 16 | - | - | - | ||||||||||||||
| Cost of revenue | 19 | - | - | - | |||||||||||||||
| Gross loss | (3 | ) | - | - | - | ||||||||||||||
| Research and development expenses, net | 1,733 | 1,883 | 1,122 | 954 | |||||||||||||||
| Marketing expenses | 989 | 324 | 543 | 109 | |||||||||||||||
| General and administrative expenses | 2,508 | 848 | 1,230 | 429 | |||||||||||||||
| Other income | (15 | ) | - | - | - | ||||||||||||||
| Operating loss | 5,218 | 3,055 | 2,895 | 1,492 | |||||||||||||||
| Financing expenses, net | 143 | 610 | 74 | 304 | |||||||||||||||
| Registration rights expense | 900 | - | 900 | - | |||||||||||||||
| Net Loss | 6,261 | 3,665 | 3,869 | 1,796 | |||||||||||||||
| Weighted average number of common shares outstanding used in computing basic and diluted loss per share | 10,175,157 | 940,028 | 10,488,647 | 940,028 | |||||||||||||||
| Basic and diluted loss per common share | (0.615 | ) | (3.898 | ) | (0.369 | ) | (1.910 | ) | |||||||||||
The accompanying notes are an integral part of the interim condensed consolidated financial statements.
| F-3 |
MOTUS GI HOLDINGS, INC.
INTERIM CONDENSED CONSOLIDATED STATEMENT OF CHANGES IN SHAREHOLDERS’ EQUITY
Expressed in U.S. dollars in thousands, except share data
| Preferred
stock - Motus Ltd. (pre- merger) | Preferred
series A stock | Common stock | ||||||||||||||||||||||||||||||||||
| Number
of shares (*) | USD | Number of shares (*) | USD | Number
of shares (*) | USD | Additional paid in capital | Accumulate deficit | Total shareholders’ equity | ||||||||||||||||||||||||||||
| Balance as of January 1, 2016 | 2,971,224 | (**) | - | - | 940,028 | (**) | 14,175 | (17,898 | ) | (3,723 | ) | |||||||||||||||||||||||||
| Conversion of convertible notes | 3,243,768 | (**) | - | - | - | - | 16,253 | - | 16,253 | |||||||||||||||||||||||||||
| Exercise of warrants | - | - | - | - | 88,748 | (**) | - | - | - | |||||||||||||||||||||||||||
| Effect of reverse recapitalization transaction | (6,214,992 | ) | (**) | 1,214,845 | (**) | 8,265,687 | 1 | 5,467 | - | 5,468 | ||||||||||||||||||||||||||
| Share-based compensation | - | - | - | - | - | - | 54 | - | 54 | |||||||||||||||||||||||||||
| Net loss | - | - | - | - | - | - | - | (8,023 | ) | (8,023 | ) | |||||||||||||||||||||||||
| Balance as of December 31, 2016 | - | - | 1,214,845 | (**) | 9,294,463 | 1 | 35,949 | (25,921 | ) | 10,029 | ||||||||||||||||||||||||||
| (Unaudited) | ||||||||||||||||||||||||||||||||||||
| Issuance of shares | - | - | 366,283 | (**) | 1,098,849 | (**) | 6,474 | - | 6,474 | |||||||||||||||||||||||||||
| Share-based compensation | - | - | - | - | 95,000 | (**) | 597 | - | 597 | |||||||||||||||||||||||||||
| Exercise of options | - | - | - | - | 754 | (**) | (**) | - | - | |||||||||||||||||||||||||||
| Net loss for the period | - | - | - | - | - | - | - | (6,261 | ) | (6,261 | ) | |||||||||||||||||||||||||
| Balance as of June 30, 2017 | - | - | 1,581,128 | (**) | 10,489,066 | 1 | 43,020 | (32,182 | ) | 10,839 | ||||||||||||||||||||||||||
| (*) | Number of shares as of January 1, 2016 has been retroactively adjusted based on the equivalent number of shares received by the accounting acquirer in the reverse recapitalization (see note 1A). |
| (**) | Represents an amount less than one thousand. |
The accompanying notes are an integral part of the interim condensed consolidated financial statements.
| F-4 |
MOTUS GI HOLDINGS, INC.
INTERIM CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
Expressed in U.S. dollars in thousands
| Six months ended June 30, | ||||||||
| 2017 | 2016 | |||||||
| Unaudited | ||||||||
| CASH FLOWS - OPERATING ACTIVITIES | ||||||||
| Net loss for the period | (6,261 | ) | (3,665 | ) | ||||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Depreciation | 25 | 18 | ||||||
| Interest and revaluation of convertible notes and other long-term liabilities | 134 | 594 | ||||||
| Share-based compensation expense | 597 | 8 | ||||||
| Changes in assets and liabilities: | ||||||||
| Decrease (increase) in other current assets | (410 | ) | 21 | |||||
| Increase in accounts receivable | (18 | ) | - | |||||
| Increase in inventory | (338 | ) | - | |||||
| Increase (decrease) in trade accounts payable | 806 | 66 | ||||||
| Increase (decrease) in other current liabilities | 804 | (59 | ) | |||||
| Net cash used in operating activities | (4,661 | ) | (3,017 | ) | ||||
| CASH FLOWS - INVESTING ACTIVITIES | ||||||||
| Acquisition of fixed assets | (473 | ) | (5 | ) | ||||
| Increase in long-term deposits | (51 | ) | 35 | |||||
| Decrease in restricted cash | 7 | (35 | ) | |||||
| Net cash used in investing activities | (517 | ) | (5 | ) | ||||
| CASH FLOWS - FINANCING ACTIVITIES | ||||||||
| Proceeds from issuance of shares, net | 6,474 | - | ||||||
| Proceeds from issuance of convertible notes | - | 2,587 | ||||||
| Net cash provided by financing activities | 6,474 | 2,587 | ||||||
| Increase (decrease) in cash and cash equivalents | 1,296 | (435 | ) | |||||
| Cash and cash equivalents at the beginning of the year | 11,644 | 1,292 | ||||||
| Cash and cash equivalents at the end of the period | 12,940 | 857 | ||||||
The accompanying notes are an integral part of the interim condensed consolidated financial statements.
| F-5 |
MOTUS GI HOLDINGS, INC.
NOTES TO THE INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
| NOTE 1 - | GENERAL |
| A. | ORGANIZATION AND BUSINESS |
Organization
Motus GI Holdings, Inc. (the “Company”) was incorporated in Delaware, U.S.A. in September 2016. The Company was established for the purpose of raising capital for the recapitalization of Motus GI Medical Technologies, Ltd. (“Motus, Ltd.”), a company incorporated in Israel and its then wholly owned subsidiary Motus GI, Inc (“Motus, Inc.”), a company incorporated in Delaware, U.S.A.
On December 22, 2016, the Company acquired (the “Recapitalization Transaction”) 100% of the outstanding shares of Motus, Ltd. pursuant to a capital stock exchange agreement dated December 1, 2016, between the Company and the shareholders of Motus, Ltd. (the “Exchange Agreement”). In exchange for the outstanding shares of Motus, Ltd., the Company issued to the shareholders of Motus, Ltd. a total of 4,000,000 shares of the Company’s common stock representing approximately 69% of the total shares then issued and outstanding after giving effect to the Recapitalization Transaction. As a result of the Recapitalization Transaction, Motus, Ltd. became a wholly owned subsidiary of the Company. The Recapitalization Transaction was accounted for as a reverse recapitalization of Motus, Ltd. As the shareholders of Motus, Ltd. received the largest ownership interest in the Company, Motus, Ltd. was determined to be the “accounting acquirer” in the reverse recapitalization. As a result, the historical financial statements of the Company were replaced with the historical financial statements of Motus, Ltd.
On December 31, 2016, the Company executed a stock purchase agreement to acquire Motus, Inc. from Motus, Ltd resulting in Motus, Inc. becoming a wholly owned subsidiary of the Company.
The Company and its subsidiaries, Motus, Ltd. and Motus, Inc., are collectively referred to as the “Company”.
Business
The Company has developed the Pure-Vu system, approved by the FDA, that cleanses the colon during a colonoscopy procedure thereby reducing the dependency on unpleasant and time consuming pre-procedural colonoscopy preparation regimens to ensure clear visualization of the colon mucosa which is critical to procedural success. The Pure-Vu system has been designed to integrate with standard colonoscopes to enable cleaning during the procedure while preserving standard procedural workflow and techniques. The Company believes the Pure-Vu system has the potential to improve procedure quality, clinical outcomes and patient satisfaction while simultaneously lowering costs and improving margins for hospitals and increasing revenues and improving margins for outpatient colonoscopy clinics.
| F-6 |
MOTUS GI HOLDINGS, INC.
NOTES TO THE INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
| NOTE 1 - | GENERAL (Cont.) |
| B. | Going Concern |
To date the Company has generated minimal revenues from its activities and has incurred substantial operating losses. Management expects the Company to continue to generate substantial operating losses and to continue to fund its operations primarily through utilization of its current financial resources, future product sales, and through additional raises of capital.
Such conditions raise substantial doubts about the Company’s ability to continue as a going concern. Management’s plan includes revenue generation through the sale of products and raising funds from outside investors. However, there is no assurance that such sale of products will occur or that outside funding will be available to the Company, will be obtained on favorable terms or will provide the Company with sufficient capital to meet its objectives. These financial statements do not include any adjustments relating to the recoverability and classification of assets, carrying amounts or the amount and classification of liabilities that may be required should the Company be unable to continue as a going concern.
| NOTE 2 - | BASIS OF PRESENTATION AND SIGNIFICANT ACCOUNTING POLICIES |
| A. | Unaudited Interim Financial Statements |
These unaudited interim consolidated financial statements have been prepared as of June 30, 2017, and for the six and three-month period then ended. Accordingly, certain information and footnote disclosures normally included in annual financial statements prepared in accordance with U.S. GAAP have been omitted. These unaudited interim consolidated financial statements should be read in conjunction with the audited financial statements and the accompanying notes of the Company for the year ended December 31, 2016.
The results of operations presented are not necessarily indicative of the results to be expected for the year ending December 31, 2017.
| B. | Significant Accounting Policies |
The significant accounting policies followed in the preparation of these unaudited interim condensed consolidated financial statements are identical to those applied in the preparation of the latest annual financial statements with the exception of the following described below.
Revenue recognition
During the first quarter of 2017, the Company began selling its products. The vast majority of the Company’s sales are expected to be achieved through the effort of its direct sales force.
In accordance with ASC Topic 605 “Revenue Recognition”, the Company recognizes revenues from sale of products when the following fundamental criteria are met: (i) persuasive evidence of an arrangement exists; (ii) delivery has occurred or services have been rendered; (iii) the price to the customer is fixed or determinable; and (iv) collection of the resulting receivable is reasonably assured. Generally, delivery occurs after products meet all of the customer’s acceptance criteria based on pre-shipment electronic, functional and quality tests.
The Company provides one-year warranty on sale of its products. No events have occurred that would indicate a need to necessitate an allowance related to warranty costs. The Company’s policy does not allow for sales returns; therefore, no allowance has been created with respect to such matter.
| F-7 |
MOTUS GI HOLDINGS, INC.
NOTES TO THE INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
| NOTE 2 - | BASIS OF PRESENTATION AND SIGNIFICANT ACCOUNTING POLICIES (Cont.) |
| C. | Recent Accounting Standards |
The Company assesses the adoption impacts of recently issued accounting standards by the Financial Accounting Standards Board on its financial statements. Following are newly issued standards or material updates to the Company’s previous assessments from its financial statements from the year ended December 31, 2016:
In May 2017, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) No. 2017-09, “Compensation-Stock Compensation (Topic 718): Scope of Modification Accounting,” which clarifies when a change to terms or conditions of a share-based payment award must be accounted for as a modification. The new guidance requires modification accounting if the vesting condition, fair value or the award classification is not the same both before and after a change to the terms and conditions of the award. The new guidance is effective on a prospective basis beginning on January 1, 2018 and early adoption is permitted. The Company does not expect the adoption of this standard to have an impact on its consolidated financial statements.
In May 2014, the FASB issued a new revenue recognition standard that will supersede current revenue recognition guidance. The core principle of the guidance is that an entity should recognize revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. The standard will be effective for the first interim period within annual periods beginning after December 15, 2017, with early adoption permitted for annual periods beginning after December 15, 2016, and can be adopted either retrospectively to each prior reporting period presented or as a cumulative effect adjustment as of the date of adoption. The Company does not expect the adoption of this standard to have a material impact on its consolidated financial statements.
| NOTE 3 - | SHARE CAPITAL |
Formation shares
During October and November 2016, the Company issued 1,650,000 shares of common stock pursuant to the formation of the Company.
Shares exchange
As detailed in Note 1, during the Recapitalization Transaction the Company issued 4,000,000 shares of common stock in exchange for 100% of the issued and outstanding and preferred shares of Motus Ltd.
| F-8 |
MOTUS GI HOLDINGS, INC.
NOTES TO THE INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
| NOTE 3 - | SHARE CAPITAL (Cont.) |
Registration Rights
In connection with the 2017 Private Placement (as defined below), the Company entered into a registration rights agreement (the “Registration Rights Agreement”) with the 2017 Private Placement investors, (the “Investors”). The Company is required to file with the SEC after the date of the final closing of the 2017 Private Placement (the “Registration Filing Date”), a registration statement covering the resale of the shares of our common stock held by the Investors (the “Investor Shares”) issued in the 2017 Private Placement, as well as the shares of our common stock underlying the Series A Convertible Preferred Stock, par value $0.0001 (the “Series A Convertible Preferred Stock”), issued in the 2017 Private Placement (together with the Investor Shares, the “Registrable Securities”). The Company is also required to use commercially reasonable efforts to have the registration statement declared effective within one hundred and fifty (150) days after the registration statement is filed (the “Effectiveness Deadline”); provided however, that if the Company signs a letter of intent or comparable agreement with an underwriter which contemplates an Initial Public Offering (“IPO”) or holds an organizational meeting for an IPO, or otherwise orally engages an underwriter to begin working with the Company towards an IPO prior to the Effectiveness Deadline (the “IPO Process Commencement Date”), then the Company shall file a joint registration statement covering the primary shares to be issued in the IPO and the resale of the Registrable Securities, and in such event the Registration Filing Date shall be extended to a date that is seventy five (75) calendar days after the IPO Process Commencement Date and the Effectiveness Deadline shall be extended to a date that is one hundred twenty (120) calendar days after the initial filing of the Registration Statement with the Commission. If the IPO is abandoned at any time, then the Registration Filing Date will be sixty (60) calendar days from the actual date of abandonment and the Effectiveness Deadline will be one hundred and fifty (150) calendar days after the date of abandonment. The Company is also required to keep the registration statement continuously effective under the Securities Act for a period of one year or for such shorter period ending on the earlier to occur of the date when all the Registrable Securities covered by the registration statement have been sold or such time as all of the Registrable Securities covered by the registration statement can be sold under Rule 144 without any volume limitations.
If this registration statement is not declared effective on or before the Effectiveness Deadline, the Company will be required to pay to each holder of Registrable Securities purchased in the 2017 Private Placement an amount in cash equal to one-half of one percent (0.5%) of such holder’s investment amount on every thirty (30) day anniversary of such Effectiveness Deadline until such failure is cured. The payment amount shall be prorated for partial thirty (30) day periods. The maximum amount of payments to be made by the Company to each Investor as the result of such failure, shall be an amount equal to six percent (6%) of each Investor’s investment amount. Notwithstanding the foregoing, no payments shall be owed with respect to any period during which all of the holder’s Registrable Securities may be sold by such holder without restriction under Rule 144. Each such payment shall be due and payable within five days after the end of each full 30-day period of the Registration Default Period. If the Company fails to pay any partial liquidated damages or refund pursuant to this Section in full within seven days after the date payable, the Company will pay interest thereon at a rate of 2% per annum.
The Company shall keep the registration statement “evergreen” for one (1) year from the date it is declared effective by the SEC or until Rule 144 of the Securities Act is available to the holders of Registrable Securities purchased in the 2017 Private Placement with respect to all of their shares, whichever is earlier. As of the issuance date of the financial statements, the registration statement was not declared effective on or before the Effectiveness Deadline. As such, the Company recorded a provision of $900,000 within other current liabilities as of June 30, 2017 with respect to the registration right penalty. In accordance with ASC 825-20, the amount was recorded in earnings as the payment became probable and the amount was reasonably estimated after the inception date of the registration rights agreement.
| F-9 |
MOTUS GI HOLDINGS, INC.
NOTES TO THE INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
| NOTE 3 - | SHARE CAPITAL (Cont.) |
Private placement
On December 22, 2016, prior to the consummation of the Recapitalization Transaction, the Company consummated a private placement transaction (the “2017 Private Placement”) as part of the Recapitalization Transaction. The 2017 Private Placement consisted of units each consisting of three-quarter of a share of common stock, par value $0.0001 and one-quarter a share of convertible preferred series A stock, par value $0.0001.
Pursuant to the 2017 Private Placement, on December 22, 2016, the Company issued 1,211,655 shares of common stock and 403,885 shares of Series A Convertible Preferred Stock for total consideration of 8,077,000.
On January 30, 2017, the Company completed the second closing of the private placement. The Company raised $2.94 million for 146,865 units consisting of 3/4 of a share of common stock and 1/4 of a share of Series A Convertible Preferred Stock.
On February 24, 2017, the Company completed the third and final closing of the private placement. The Company raised $4.4 million for 219,418 units consisting of 3/4 of a share of common stock and 1/4 of a share of Series A Convertible Preferred Stock.
Each share of Series A Convertible Preferred Stock is initially convertible at the option of the holder into one share of common stock. Each share of Series A Convertible Preferred Stock will automatically convert into one share of common stock at the earliest to occur of (a) three years from the initial closing of the 2017 Private Placement or (b) notice by the Company to the holders of Series A Convertible Preferred Stock that the Company has elected to convert all outstanding shares (“Mandatory Conversion Date “). Holders of the Series A Convertible Preferred Stock will not be entitled to receive dividends. The preferred stockholders will vote on an as-converted basis with the common stockholders, and upon any liquidation, dissolution or winding-up of the Company, the holders of the Series A Convertible Preferred Stock will be entitled to receive, for each share of preferred stock an amount equal to the stated value plus any accrued and unpaid dividends thereon before any distribution or payment may be made to the common stockholders.
The Series A Convertible Preferred Stock includes the right, as a group, to receive: (i) a percentage of the net sales of the Pure-Vu™ system, and (ii) a percentage of the proceeds, if any, received by the Company in connection with the licensing of the Pure-Vu™ system (“Royalty Payment Rights”). See Note 5 for additional information.
Exchange of convertible notes
On December 22, 2016, Motus Ltd. was obligated with respect to convertible notes in the amount of $14,596,683, inclusive of accrued interest. In connection with the Recapitalization Transaction, the Company exchanged the convertible notes for approximately 2,432,808 shares of common stock and 810,960 shares of Series A Convertible Preferred Stock.
Convertible notes warrants
As a result of the Recapitalization Transaction, the Company issued 907,237 convertible note warrants (the “CNA Warrants”) to replace the warrants previously issued to the convertible note holders. The five-year CNA Warrants are exercisable for the Company’s common stock at an exercise price of $5.00 per share.
| F-10 |
MOTUS GI HOLDINGS, INC.
NOTES TO THE INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
| NOTE 4 - | SHARE-BASED COMPENSATION |
Employee stock option grant
The Company has one option plan that was approved in 2009. This plan was adjusted in 2016 following the reverse recapitalization transaction on December 22, 2016.
According to the original plan, employees and service providers were entitled to receive options to purchase common shares of Motus Ltd. Following the recapitalization, the options granted will entitle their holder to purchase shares of common stock of the Company. As a result, the number of options and exercise price per share were adjusted in a technical manner such that there was no change in the fair value of the awards under the adjusted plan. All number of options and exercise prices in this Note have been restated to reflect the adjusted option plan.
From 2012 through 2015, the Company granted its employees, not including its CEO, options to purchase an aggregate of 90,108 shares of common stock of the Company at an exercise price ranging from $2.38 to $2.52 per share. The options will expire 10 years from the date of issuance. Some of the options have a vesting period of 3 years, while others are upon the achievement of certain milestones. The remaining unvested shares will vest upon 1) the Company’s obtainment of CE approval of its system; and 2) upon the enrollment of the first patient in a post market study with a “prep-less” indication, or the sale of the first 1,000 disposables.
On April 2, 2014, the Company granted its CEO options to purchase 67,238 shares of the Company’s common stock. Of the total options granted, 48,527 options will vest upon the achievement of certain milestones, as detailed, above and additional milestones including the gross return in multiples on preferred A shares. The remaining 18,711 options will vest over a period of 3 years. The exercise price of the options are $2.38 per share.
On May 4, 2017, the Company’s Board of Directors approved the issuance of 1,726,769 options to directors, employees, and consultants. The additional options that were granted have an exercise price of $5.00 and vest in accordance with the terms of the option agreements.
Among this grant, the Company’s CEO received options to purchase 511,113 shares of the Company’s common stock. Fifty-three percent (53%) of the options subject to the option were fully vested immediately upon grant, forty percent (40%) of the options will vest in a series of twelve (12) successive equal quarterly installments upon the CEO’s completion of each successive calendar quarter of active service over the three (3) year period measured from the date of grant, and seven percent (7%) of the options will vest on December 22, 2019, provided that the CEO remains a service provider to the Company through each applicable vesting date. Additionally, the Company’s former CFO as of the grant date, received options to purchase 154,227 shares of the Company’s common stock. A portion of the options vested on the grant date and the remaining options will vest over a period of 3 years.
On May 4, 2017, the Company’s Board of Directors approved unrestricted stock awards for the issuance of 5,000 shares of its common stock to employees of the Company, under the 2016 Equity Incentive Plan.
| F-11 |
MOTUS GI HOLDINGS, INC.
NOTES TO THE INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
| NOTE 4 - | SHARE-BASED COMPENSATION (Cont.) |
Employee stock option grant (Cont.)
A summary of the Company’s option activity related to options to employees and related information is as follows:
| For the six months ended June 30, 2017 | ||||||||||||
| Shares | Weighted Average Exercise Price | Weighted average remaining contractual term (years) | ||||||||||
| Outstanding at beginning of period | 110,711 | 2.42 | 8 | |||||||||
| Granted | 1,113,269 | 5 | 10 | |||||||||
| Exercised | (754 | ) | - | - | ||||||||
| Cancelled | - | - | - | |||||||||
| Outstanding at end of period | 1,223,226 | 4.77 | 9.8 | |||||||||
The number of options that had vested as of June 30, 2017 was 456,181.
The aggregate intrinsic value (the difference between the fair market value of the Company’s common stock on June 30, 2017, and the exercise price, multiplied by the number of in-the-money options on that date) was $179,415.
Compensation expense recorded by the Company in respect of options granted in accordance with ASC 718-10 for the period ended June 30, 2017 and 2016 amounted to $316,000 and $8,000, respectively. The compensation expenses for the six months ended June 30 2017 were recorded in the statement of comprehensive loss as follows: $236,000 in general and administrative, $37,000 in sales and marketing expenses and $43,000 in research and development expenses. The compensation expenses for the six months ended June 30 2016 were recorded in the statement of comprehensive loss as follows: $4,000 in general and administrative expenses and $4,000 in research and development expenses.
The fair value of the stock options granted on May 4, 2017 was estimated at the date of grant using the Black-Scholes options pricing model with the following weighted-average assumptions:
| Expected volatility | 60 | % | ||
| Dividend Yield | 0 | % | ||
| Risk-free interest | 2.36 | % | ||
| Expected life of up to (years) | 5 |
Options and warrants to service providers
The Company accounts for options to purchase common stock issued to non-employees using the guidance of ASC 505-50, “Equity-Based Payments to Non-Employees”, whereby the fair value of such options is determined at the earlier of the date at which the non-employee’s performance is completed or a performance commitment is reached.
In 2012 and 2014, the Company granted 7,510 and 7,509 options to its service providers, respectively, to purchase shares of the Company’s common stock at an exercise price of $2.38 per share. The options vest over a period of 3 years and will expire 10 years from the date of issuance.
| F-12 |
MOTUS GI HOLDINGS, INC.
NOTES TO THE INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
| NOTE 4 - | SHARE-BASED COMPENSATION (Cont.) |
Options and warrants to service providers (Cont.)
In January 2017, the Company signed a consulting service agreement by which it granted the service provider an option to purchase 100,000 shares of the Company’s common stock in exchange for its services. The options were fully vested as of the signing date of the agreement and may be exercised during a period of 5 years from issuance at an exercise price of $5.00 per share. As of June 30, 2017, the Company recorded an expense in the amount of $40,000 with respect to this agreement.
In March 2017, the Company signed a consulting service agreement by which it granted the service provider 90,000 shares of the Company’s common stock, subject to a lock-up agreement, and a warrant to purchase 30,000 shares of the Company’s common stock at an exercise price of $8.00 per share, in exchange for its services. The warrant will vest and become exercisable as follows: (i) 7,500 warrant shares will become exercisable on December 27, 2017, (ii) 7,500 warrant shares will become exercisable on the six month anniversary of the date the Securities and Exchange Commission declares the Company’s Registration Statement on Form S-1 (the “Registration Statement Effectiveness Date”), and (iii) 15,000 warrant shares will become exercisable on the twelve month anniversary of the Registration Statement Effectiveness Date. The warrants are exercisable for a period of 5 years from the signing of the agreement. As of June 30, 2017, the Company recorded an expense in the amount of $215,000 with respect to this agreement. The share-based compensation expense previously recognized on unvested warrants to this service provider were revalued as of June 30, 2017 using the same assumptions as the options granted to employees and service providers in May 2017.
As part of the 1,726,769 options granted on May 4, 2017, Directors of the Company received options to purchase 482,500 shares of the Company’s common stock. The options will vest on the first and second anniversary of the grant date contingent upon continued services as director of the Company. As of June 30, 2017, the Company had not incurred any expenses with regards to these options.
Additionally, the Company granted options to purchase 31,000 common shares of the Company to two services providers in exchange for consulting services. A portion of the options vested on the grant date and the remaining options will vest in a series of twelve equal, quarterly installments contingent upon providing continued service as of each quarter over a three-year period from the grant date. The exercise price of the options are $5.00 and will expire 10 years from the grant date.
In connection with the 2017 Private Placement, the Company issued 403,632 warrants to purchase 403,632 shares of the Company’s common stock to the placement agent at an exercise price equal to the fair value of the common stock on the grant date. These warrants are exercisable for a period of 5 years from the grant date and provide for a cashless exercise.
Compensation expense recorded by the Company in respect of options granted in accordance with ASC 505-50 for the period ended June 30, 2017 amounted to $254,000. This amount was recorded as general and administrative expenses.
| NOTE 5 - | OTHER LONG-TERM LIABILITIES |
As a part of the 2017 Private Placement, the Company issued Series A Convertible Preferred Stock which entitle its holders, in aggregate, to a royalty in an amount of:
| ● | 3% of net sales subject to a maximum in any calendar year equal to the total dollar amount of units closed on in the private placement offering; and | |
| ● | 5% of licensing proceeds subject to a maximum in any calendar year equal to the total dollar amount of units closed on in the private placement. |
| F-13 |
MOTUS GI HOLDINGS, INC.
NOTES TO THE INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
| NOTE 5 - | OTHER LONG-TERM LIABILITIES (Cont.) |
Even if converted pursuant to the mandatory conversion as defined in Note 3 above, the royalty right certificate will remain in the hands of the holder in addition to the common stock to be issued following conversion. If converted at the option of the holder prior to the mandatory conversion, the holder will lose all rights to future royalty payments.
In addition, the Company issued royalty rights certificates to the placement agent, and its designees, with substantially similar terms as those of the Series A Convertible Preferred Stock, which grants the placement agent, and its designees the right, in the aggregate, to receive 10% of the amount of payments paid to the holders of the Series A Convertible Preferred Stock.
The royalty rights certificates were recorded as a liability at fair value in the consolidated financial statements with changes in the fair value recorded in profit and loss.
Activity of all royalty right liabilities, which are measured on a recurring basis, was as follow for the period ended June 30, 2017:
| Other long-term liabilities | ||||
| As of December 31, 2016 | $ | 1,410 | ||
| Revaluation of liabilities | 134 | |||
| As of June 30, 2017 | 1,544 | |||
The Company measures the fair value of the liabilities using the discounted cash flow method using level 3 assumptions, namely a discount rate of 20%. The fair value at inception was allocated to the royalty rights and the residual value was allocated to the preferred shares and recorded as equity.
In accordance with ASC-820-10-50-2(g), the Company performed a sensitivity analysis of the liability, which was classified as a level 3 financial instrument. The Company recalculated the fair value of the liability by applying a +/- 2% change to the input variable in the discounted cash flow model; the discount rate. A 2% decrease in the discount rate would increase the liability by approximately $167,000; a 2% increase in the discount rate would decrease the liability by approximately $144,000.
| NOTE 6 - | COMMITMENTS AND CONTINGENCIES |
On April 13, 2017, the Company entered into a lease for a facility in Fort Lauderdale, Florida, which the Company intends to begin occupying in October 2017. The facility will be initially 4,554 square feet, which will increase to 6,390 square feet by the second year of the lease. The term will run for seven years and two months from the date the Company begins to occupancy the facility. Annual base rent is initially $156,555 per year, subject to annual increases of 2.75%.
The Company currently has a lease agreement for its facilities in Israel through December 31, 2019. The annual lease fees are $82,000. The Company has an option to renew the lease agreement for three more years after the initial term period ends. The annual lease fees will increase by 4% at the option period.
| NOTE 7 - | SUBSEQUENT EVENTS |
On August 16, 2017, the Company hired a new CFO. Pursuant to the terms of the employment agreement, the CFO will be granted options to purchase 240,000 common shares of the Company. The options will vest over a three year period on a quarterly basis and the exercise price will be equal to the fair market value on the grant date, as determined by the Board of Directors.
| F-14 |
MOTUS GI HOLDINGS, INC.
NOTES TO THE INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
| NOTE 7 - | SUBSEQUENT EVENTS (Cont.) |
The Company’s former CFO’s share based payment awards granted in May 2017 were forfeited as of September 7, 2017.
On September 29, 2017, the Board of Directors approved the repricing of the May 4, 2017 options awarded from an exercise price of $5.00 to $4.50 per share. This change will be accounted for as a modification of a share-based payment award.
| F-15 |

REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of
Motus GI Holdings, Inc.
We have audited the accompanying consolidated balance sheets of Motus GI Holdings, Inc. and its subsidiaries (the “Company”) as of December 31, 2016 and December 31, 2015 and the related consolidated statements of comprehensive loss, changes in stockholders’ equity, and cash flows for the years ended December 31, 2016 and December 31, 2015. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the financial statements based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audit provide a reasonable basis for our opinion.
In our opinion, based on our audit, such consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2016 and December 31, 2015 and the results of its operations and cash flows for the years ended December 31, 2016 and December 31, 2015 in conformity with accounting principles generally accepted in the United States of America.
| /s/ Brightman Almagor Zohar & Co. | |
| Brightman Almagor Zohar & Co. | |
| Certified Public Accountants | |
| Member of Deloitte Touche Tohmatsu Limited | |
| Tel Aviv, Israel | |
| April 7, 2017 |
| F-16 |
MOTUS GI HOLDINGS, INC.
Expressed in U.S. dollars in thousands
| As of December 31, | ||||||||||
| Note | 2016 | 2015 | ||||||||
| ASSETS | ||||||||||
| Current assets | ||||||||||
| Cash and cash equivalents | 2E | 11,644 | 1,292 | |||||||
| Restricted cash | 7 | - | ||||||||
| Inventory | 4 | 81 | - | |||||||
| Other current assets | 3 | 263 | 180 | |||||||
| Total current assets | 11,995 | 1,472 | ||||||||
| Fixed assets, net | 5 | 141 | 157 | |||||||
| Long-term deposits | 55 | 86 | ||||||||
| 196 | 243 | |||||||||
| Total Assets | 12,191 | 1,715 | ||||||||
| LIABILITIES AND SHAREHOLDERS’ EQUITY | ||||||||||
| Current liabilities | ||||||||||
| Trade accounts payable | 107 | 462 | ||||||||
| Other current liabilities | 6 | 645 | 236 | |||||||
| 752 | 698 | |||||||||
| Convertible notes | 10 | - | 4,740 | |||||||
| Other long-term liabilities | 8 | 1,410 | - | |||||||
| Shareholders’ equity (deficit) (**) | 8 | |||||||||
| Common stock - $0.0001 par value | 1 | (*) | ||||||||
| Authorized: 50,000,000 and 9,904,081 as of December 31, 2016 and December 31, 2015, respectively | ||||||||||
| Issued and outstanding: 9,294,463 and 940,028 as of December 31, 2016 and December 31, 2015, respectively | ||||||||||
| Preferred series A stock - $0.0001 par value (Motus Holdings) | (*) | - | ||||||||
| Authorized: 2,000,000 as of December 31, 2016 | ||||||||||
| Issued and outstanding: 1,214,845 as of December 31, 2016 | ||||||||||
| Preferred stock - $0.0001 par value (Motus Holdings) | - | - | ||||||||
| Authorized: 8,000,000 as of December 31, 2016 | ||||||||||
| Issued and outstanding: 0 as of December 31, 2016 | ||||||||||
| Preferred A stock - $0.0001 par value (Motus Ltd.) | - | (*) | ||||||||
| Authorized: 7,262,992 as of December 31, 2015 | ||||||||||
| Issued and outstanding: 2,971,224 as of December 31, 2015 | ||||||||||
| Additional paid-in capital | 35,949 | 14,175 | ||||||||
| Accumulated deficit | (25,921 | ) | (17,898 | ) | ||||||
| Total shareholders’ equity (deficit) | 10,029 | (3,723 | ) | |||||||
| Total liabilities and shareholders’ equity | 12,191 | 1,715 | ||||||||
| (*) | Represents an amount less than one thousand. |
| (**) | Number of shares as of December 31, 2015 has been retroactively adjusted based on the equivalent number of shares received by the accounting acquirer in the reverse recapitalization (see note 1A). |
The accompanying notes are an integral part of the consolidated financial statements.
| F-17 |
MOTUS GI HOLDINGS, INC.
CONSOLIDATED STATEMENTS OF COMPREHENSIVE LOSS
Expressed in U.S. dollars in thousands, except share and per share information
| Year ended December 31, | ||||||||||||
| Note | 2016 | 2015 | ||||||||||
| Research and development expenses, net | 11 | 3,079 | 3,160 | |||||||||
| Marketing expenses | 12 | 1,034 | 415 | |||||||||
| General and administrative expenses | 13 | 1,894 | 1,750 | |||||||||
| Operating loss | 6,007 | 5,325 | ||||||||||
| Financing expenses, net | 14 | 1,966 | 637 | |||||||||
| Loss before income taxes | 7,973 | 5,962 | ||||||||||
| Income tax expenses | 15 | 50 | 29 | |||||||||
| Net loss for the year | 8,023 | 5,991 | ||||||||||
| Weighted average number of common shares outstanding used in computing basic and diluted loss per share (*) | 16 | 1,146,028 | 940,028 | |||||||||
| Basic and diluted loss per common share | (7.00 | ) | (6.37 | ) | ||||||||
| (*) | Number of shares has been retroactively adjusted based on the equivalent number of shares received by the accounting acquirer in the reverse recapitalization (see note 1A). |
The accompanying notes are an integral part of the consolidated financial statements.
| F-18 |
MOTUS GI HOLDINGS, INC.
CONSOLIDATED STATEMENT OF CHANGES IN SHAREHOLDERS’ EQUITY (DEFICIT)
Expressed in U.S. dollars in thousands, except share data
| Preferred stock - | ||||||||||||||||||||||||||||||||||||
Motus Ltd. (pre- merger) | Preferred series A stock | Common Stock | Total | |||||||||||||||||||||||||||||||||
| Additional | shareholders’ | |||||||||||||||||||||||||||||||||||
| Number of | Number of | Number of | paid in | Accumulated | equity | |||||||||||||||||||||||||||||||
| shares (*) | USD | shares (*) | USD | shares (*) | USD | capital | Deficit | (deficit) | ||||||||||||||||||||||||||||
| Balance as of January 1, 2015 | 2,630,446 | (**) | - | - | 940,028 | (**) | 12,650 | (11,907 | ) | 743 | ||||||||||||||||||||||||||
| Issuance of preferred shares | 340,778 | (**) | - | - | - | - | 1,514 | - | 1,514 | |||||||||||||||||||||||||||
| Share-based compensation | - | - | - | - | - | - | 11 | - | 11 | |||||||||||||||||||||||||||
| Loss for the year | - | - | - | - | - | - | - | (5,991 | ) | (5,991 | ) | |||||||||||||||||||||||||
| Balance as of December 31, 2015 | 2,971,224 | (**) | - | - | 940,028 | (**) | 14,175 | (17,898 | ) | (3,723 | ) | |||||||||||||||||||||||||
| Conversion of convertible notes | 3,243,768 | (**) | - | - | - | - | 16,253 | - | 16,253 | |||||||||||||||||||||||||||
| Exercise of warrants | - | - | - | - | 88,748 | (**) | - | - | - | |||||||||||||||||||||||||||
| Effect
of reverse recapitalization transaction | (6,214,992 | ) | (**) | 1,214,845 | (**) | 8,265,687 | 1 | 5,467 | - | 5,468 | ||||||||||||||||||||||||||
| Share-based compensation | - | - | - | - | - | - | 54 | - | 54 | |||||||||||||||||||||||||||
| Net loss for the year | - | - | - | - | - | - | - | (8,023 | ) | (8,023 | ) | |||||||||||||||||||||||||
| Balance as of December 31, 2016 | - | - | 1,214,845 | (**) | 9,294,463 | 1 | 35,949 | (25,921 | ) | 10,029 | ||||||||||||||||||||||||||
| (*) | Number of shares as of December 31, 2015 has been retroactively adjusted based on the equivalent number of shares received by the accounting acquirer in the reverse recapitalization (see note 1A). |
| (**) | Represents an amount less than one thousand. |
The accompanying notes are an integral part of the consolidated financial statements.
| F-19 |
MOTUS GI HOLDINGS, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
Expressed in U.S. dollars in thousands
| Year ended December 31, | ||||||||
| 2016 | 2015 | |||||||
| CASH FLOWS - OPERATING ACTIVITIES | ||||||||
| Loss for the year | (8,023 | ) | (5,991 | ) | ||||
| Adjustments to reconcile loss to net cash from operating activities | ||||||||
| (Appendix A) | 1,897 | 1,068 | ||||||
| Net cash used in operating activities | (6,126 | ) | (4,923 | ) | ||||
| CASH FLOWS - INVESTING ACTIVITIES | ||||||||
| Acquisition of fixed assets | (30 | ) | (68 | ) | ||||
| Decrease (Increase) in long-term deposit | 31 | (86 | ) | |||||
| Decrease (Increase) in restricted cash | (7 | ) | - | |||||
| Net cash used in investing activities | (6 | ) | (154 | ) | ||||
| CASH FLOWS - FINANCING ACTIVITIES | ||||||||
| Proceeds from issuance of shares, net | - | 1,514 | ||||||
| Cash acquired in connection with the reverse recapitalization, net (see note 1A) | 6,878 | - | ||||||
| Proceeds from issuance of convertible notes | 9,606 | 4,107 | ||||||
| Net cash provided by financing activities | 16,484 | 5,621 | ||||||
| Increase in cash and cash equivalents | 10,352 | 544 | ||||||
| Cash and cash equivalents at the beginning of the year | 1,292 | 748 | ||||||
| Cash and cash equivalents at the end of the year | 11,644 | 1,292 | ||||||
| Significant Non-Cash Transactions: | ||||||||
| Non-cash financing and investing activities: | ||||||||
| Convertible notes exchanged for common and preferred stock | $ | 16,253 | ||||||
The accompanying notes are an integral part of the consolidated financial statements.
| F-20 |
MOTUS GI HOLDINGS, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
Expressed in U.S. dollars in thousands
| Appendix A - | Adjustments to reconcile loss to net cash from operating activities: |
| Year ended December 31, | ||||||||
| 2016 | 2015 | |||||||
| Items not involving cash flows: | ||||||||
| Depreciation | 46 | 45 | ||||||
| Interest and revaluation of convertible notes | 1,907 | 633 | ||||||
| Share-based compensation expense | 54 | 11 | ||||||
| Changes in operating assets and liabilities: | ||||||||
| Decrease (increase) in other current assets | (83 | ) | 49 | |||||
| Increase in inventory | (81 | ) | - | |||||
| Increase (decrease) in trade accounts payable | (355 | ) | 371 | |||||
| Increase (decrease) in other payables | 409 | (41 | ) | |||||
Total adjustments to reconcile loss to net cash from operating activities |
1,897 | 1,068 | ||||||
The accompanying notes are an integral part of the consolidated financial statements.
| F-21 |
MOTUS GI HOLDINGS, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
| NOTE 1 - | GENERAL |
| A. | ORGANIZATION AND BUSINESS |
| Organization | |
| Motus GI Holdings, Inc. (the “Company”) was incorporated in Delaware, U.S.A. in September 2016. The Company was established for the purpose of raising capital for the recapitalization of Motus GI Medical Technologies, Ltd. (“Motus, Ltd.”), a company incorporated in Israel and its then wholly owned subsidiary Motus GI, Inc. (“Motus, Inc.”), a company incorporated in Delaware, U.S.A. | |
| On December 22, 2016, the Company acquired (the “Recapitalization Transaction”) 100% of the outstanding shares of Motus, Ltd. pursuant to a capital stock exchange agreement dated December 1, 2016, between the Company and the shareholders of Motus, Ltd. (the “Exchange Agreement”). In exchange for the outstanding shares of Motus, Ltd., the Company issued to the shareholders of Motus, Ltd. a total of 4,000,000 of the Company’s common shares representing approximately 69% of the total shares then issued and outstanding after giving effect to the Recapitalization Transaction. As a result of the Recapitalization Transaction, Motus, Ltd. became a wholly owned subsidiary of the Company. The Recapitalization Transaction was accounted for as a reverse recapitalization of Motus, Ltd. As the shareholders of Motus, Ltd. received the largest ownership interest in the Company, Motus, Ltd. was determined to be the “accounting acquirer” in the reverse recapitalization. As a result, the historical financial statements of the Company were replaced with the historical financial statements of Motus, Ltd. | |
| Cash acquired in connection with the reverse capitalization per the statement of cash flows refers to the total net cash from the first closing of the 2017 Private Placement (gross proceeds of $8.077 million less approximately $1.2 million in issuance costs). A schedule showing assets acquired and liabilities assumed is as follows: |
| Year ended December 31, 2016 | ||||
| Other long-term liabilities | $ | 1,410 | ||
| Reverse recapitalization effect on equity | $ | 5,468 | ||
| Cash acquired upon reverse recapitalization | $ | 6,878 | ||
| On December 31, 2016, the Company executed a stock purchase agreement to acquire Motus, Inc. from Motus, Ltd resulting in Motus, Inc. becoming a wholly owned subsidiary of the Company. | |
| The Company and its subsidiaries, Motus, Ltd. and Motus, Inc., are collectively referred to as the “Company”. | |
| Business | |
| The Company has developed the Pure-Vu system, approved by the FDA, that cleanses the colon during a colonoscopy procedure thereby reducing the dependency on pre-procedural colonoscopy preparation regimens to ensure clear visualization of the colon mucosa which is critical to procedural success. The Pure-Vu system has been designed to integrate with standard colonoscopes to enable cleaning during the procedure while preserving standard procedural workflow and techniques. The Pure-Vu system, which received FDA 510(k) clearance in September 2016, has the potential to give the gastroenterologist flexibility in instructing patients how to prepare for a colonoscopy, which currently requires extensive, time consuming and unpleasant bowel preparation regimens. The Company believes the Pure-Vu system has the potential to improve procedure quality, clinical outcomes and patient satisfaction while simultaneously lowering costs and improving margins for hospitals and increasing revenues and improving margins for outpatient colonoscopy clinics. |
| F-22 |
MOTUS GI HOLDINGS, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
| NOTE 1 - | GENERAL (Cont.) |
| B. | Risk factors |
| To date the Company has not yet generated revenues from its operations. As of the date of issuance of these financial statements, the Company has a cash and cash equivalent balance of approximately $15.8 million, which the Company believes is sufficient to fund its operations for more than 12 months from the date of issuance of these financial statements and sufficient to fund its operations necessary to continue development activities of its current proposed products. The Company plans to continue to fund its current operations as well as other development activities relating to additional product candidates, through future issuances of either debt and/or equity securities and possibly additional grants from the Israeli Innovation Authority. |
| NOTE 2 - | SIGNIFICANT ACCOUNTING POLICIES |
The significant accounting policies used in the preparation of the financial statements are as follows:
| A. | Basis of presentation | |
| The financial statements have been prepared in conformity with accounting principles generally accepted in the United States of America. | ||
| B. | Use of estimates | |
| The preparation of consolidated financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities as of the date of the financial statements, and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates. | ||
| C. | Functional currency and foreign currency translation | |
| The functional currency of the Company is the U.S dollar (“dollar”) since the dollar is the currency of the primary economic environment in which the Company has operated and expects to continue to operate in the foreseeable future. | ||
| Transactions and balances denominated in dollars are presented at their original amounts. Transactions and balances denominated in foreign currencies have been re-measured to dollars in accordance with the provisions of ASC 830-10, “Foreign Currency Translation”. | ||
| All transaction gains and losses from re-measurement of monetary balance sheet items denominated in non-dollar currencies are reflected in the statement of operations as financial income or expenses, as appropriate. |
| F-23 |
MOTUS GI HOLDINGS, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
| NOTE 2 - | SIGNIFICANT ACCOUNTING POLICIES (Cont.) |
| D. | Consolidation | |
| The condensed consolidated financial statements include the accounts of the Company and its wholly owned subsidiaries, Motus Ltd., an Israel corporation, which has operations in Haifa, Israel, and Motus Inc., a Delaware corporation, which has operations in the U.S. All inter-company accounts and transactions have been eliminated in consolidation. | ||
| E. | Cash and cash equivalents, net | |
| The Company considers all highly liquid short-term investments purchased with an original maturity date of three months or less to be cash equivalents. The Company had approximately $11.6 million and $1.3, on deposit in bank operating accounts at December 31, 2016 and 2015, respectively. | ||
| F. | Fair value of financial instruments | |
| The carrying values of cash and cash equivalents, other current assets, accounts payable, and other current liabilities approximate their fair value due to the short-term maturity of these instruments. | ||
| The Company measures the fair value of certain of its financial instruments (such as the liability related to royalty payments) on a recurring basis. The method of determining the fair value of other long-term liabilities is discussed in Note 8. | ||
| A fair value hierarchy is used to rank the quality and reliability of the information used to determine fair values. Financial assets and liabilities carried at fair value will be classified and disclosed in one of the following three categories: | ||
| Level 1 - Quoted prices (unadjusted) in active markets for identical assets and liabilities. | ||
| Level 2 - Inputs other than Level 1 that are observable, either directly or indirectly, such as unadjusted quoted prices for similar assets and liabilities, unadjusted quoted prices in the markets that are not active, or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities. | ||
| Level 3 - Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities. | ||
| G. | Inventory | |
| Inventories are stated at lower of cost or market using the weighted average cost method and are evaluated at least annually for impairment. Inventories at December 31, 2016 consisted of components to be used in the manufacturing of inventory. Write-downs for potentially obsolete or excess inventory are made based on management’s analysis of inventory levels, historical obsolescence and future sales forecasts. There was no inventory at December 31, 2015. |
| F-24 |
MOTUS GI HOLDINGS, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
| NOTE 2 - | SIGNIFICANT ACCOUNTING POLICIES (Cont.) |
| H. | Fixed assets, net | |
| Fixed assets are stated at cost less accumulated depreciation. Depreciation is calculated based on the straight-line method, at annual rates reflecting the estimate useful lives of the related assets, as follows: |
| % | ||||
| Computers and software | 33 | |||
| Laboratory equipment | 15 | |||
| Leasehold improvements | 10 | |||
| I. | Stock-based compensation | |
| The Company applies ASC 718-10, “Share-Based Payment,” which requires the measurement and recognition of compensation expenses for all share-based payment awards made to employees and directors including employee stock options under the Company’s stock plans based on estimated fair values. | ||
| ASC 718-10 requires companies to estimate the fair value of equity-based payment awards on the date of grant using an option-pricing model. The value of the portion of the award that is ultimately expected to vest is recognized as an expense over the requisite service periods in the Company’s statement of operations. | ||
| The Company recognizes compensation expenses for the value of non-employee awards, which have graded vesting, based on the straight-line method over the requisite service period of each award, net of estimated forfeitures. | ||
| The Company estimates the fair value of stock options granted as equity awards using a Black-Scholes options pricing model. The option-pricing model requires a number of assumptions, of which the most significant are share price, expected volatility and the expected option term (the time from the grant date until the options are exercised or expire). Expected volatility is estimated based on volatility of similar companies in the technology sector. The Company has historically not paid dividends and has no foreseeable plans to issue dividends. The risk-free interest rate is based on the yield from governmental zero-coupon bonds with an equivalent term. The expected option term is calculated for options granted to employees and directors using the “simplified” method. Grants to non-employees are based on the contractual term. Changes in the determination of each of the inputs can affect the fair value of the options granted and the results of operations of the Company. |
| F-25 |
MOTUS GI HOLDINGS, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
| NOTE 2 - | SIGNIFICANT ACCOUNTING POLICIES (Cont.) |
| J. | Basic and diluted net loss per share | |
| Basic loss per share is computed by dividing the net loss, as adjusted to include preferred shares dividend participation rights by the weighted average number of ordinary shares outstanding during the year. Shares and preferred shares contingently issuable for little or no cash are included in basic loss per share on an as issued basis. | ||
| Diluted loss per share is computed by dividing the net loss, as adjusted to include preferred shares dividend participation rights of preferred shares outstanding during the year as well as of preferred shares that would have been outstanding if all potentially dilutive preferred shares had been issued, by the weighted average number of ordinary shares outstanding during the year, plus the number of ordinary shares that would have been outstanding if all potentially dilutive ordinary shares had been issued, using the treasury stock method, in accordance with ASC 260-10 “Earnings per Share”. | ||
| The weighted average number of common shares outstanding has been retroactively restated for the equivalent number of common shares received by the accounting acquirer as a result of the reverse recapitalization as if these common shares had been outstanding as of the beginning of the earliest period presented. | ||
| Potentially dilutive common shares were excluded from the calculation of diluted loss per share for all periods presented due to their anti-dilutive effect. The number of anti-dilutive common shares which were excluded from the calculation is 2,251,148 and 3,263,717 for 2016 and 2015, respectively. | ||
| K. | Research and development costs, net | |
| Research and development expenses are charged to the statement of comprehensive loss as incurred. Grants received for funding of approved research and development projects are recognized at the time the Company is entitled to such grants, on the basis of the costs incurred and applied as a deduction from the research and development expenses. | ||
| L. | Patent costs | |
| Costs incurred in connection with acquiring patent rights and the protection of proprietary technologies are charged to expense as incurred. | ||
| M. | Convertible notes | |
| Convertible notes with characteristics of both liabilities and equity are classified as either debt or equity based on the characteristics of their monetary value, with convertible notes classified as debt being measured at fair value, in accordance with ASC 480-10, “Accounting for Certain Financial instruments with Characteristics of both Liabilities and Equity”. |
| F-26 |
MOTUS GI HOLDINGS, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
| NOTE 2 - | SIGNIFICANT ACCOUNTING POLICIES (Cont.) |
| N. | Liabilities due to termination of employment agreements | |
| Under Israeli employment laws, employees of Motus Ltd. are included under Article 14 of the Severance Compensation Act, 1963 (“Article 14”) for a portion of their salaries. According to Article 14, these employees are entitled to monthly deposits made by Motus Ltd. on their behalf with insurance companies. | ||
| Payments in accordance with Article 14 release Motus Ltd. from any future severance payments (under the Israeli Severance Compensation Act, 1963) with respect of those employees. The aforementioned deposits are not recorded as an asset in the Company’s balance sheet, and there is no liability recorded as the Company does not have a future obligation to make any additional payments. | ||
| O. | Reclassification | |
| Certain prior year amounts have been reclassified to conform to the current year presentation. | ||
| P. | Transaction Costs | |
| Transaction costs incurred in the Recapitalization Transaction were charged directly to equity to the extent of cash and net other current assets acquired. | ||
| Q. | Recent accounting standards | |
| In May 2014, the Financial Accounting Standards Board (the “FASB”) issued a new standard to achieve a consistent application of revenue recognition within the U.S., resulting in a single revenue model to be applied by reporting companies under U.S. generally accepted accounting principles. Under the new model, recognition of revenue occurs when a customer obtains control of the promised goods or services in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. In addition, the new standard requires that reporting companies disclose the nature, amount, timing, and uncertainty of revenue and cash flows arising from contracts with customers. The new standard is effective for us beginning in the first quarter of 2018; early adoption is prohibited. The new standard is required to be applied retrospectively to each prior reporting period presented or retrospectively with the cumulative effect of initially applying it recognized at the date of initial application. As the Company has not incurred revenues to date, it is unable to determine the expected impact the new standard will have on its consolidated financial statements. | ||
| In January 2016, the FASB issued ASU 2016-01 “Recognition and Measurement of Financial Assets and Financial Liabilities”, which provides targeted improvements to the recognition, measurement, presentation and disclosure of financial assets and financial liabilities. Specific accounting areas addressed include, equity investments, financial liabilities reported under the fair value option and valuation allowance assessment resulting from unrealized losses on available-for-sale securities. The standard also changes certain presentation and disclosure requirements for financial instruments. This ASU is effective for the Company in its first quarter of fiscal year 2019. Early adoption, with certain exceptions, is not permitted. The Company does not expect that the adoption of this standard will have a significant impact on the financial position or results of operations. |
| F-27 |
MOTUS GI HOLDINGS, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
Expressed in U.S. dollars in thousands
| NOTE 2 - | SIGNIFICANT ACCOUNTING POLICIES (Cont.) |
| Q. | Recent accounting standards (Cont.) | |
| In February 2016, the FASB issued Accounting Standards Update No. 2016-02, Leases (Topic 842) (“ASU 2016-02”), which amends, among other things, the existing guidance by requiring lessees to recognize lease assets (right-to-use) and liabilities (for reasonably certain lease payments) arising from operating leases on the balance sheet. For leases with a term of twelve months or less, ASU 2016-02 permits an entity to make an accounting policy election to recognize such leases as lease expense, generally on a straight-line basis over the lease term. ASU 2016-02 is effective for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2018 using a modified retrospective approach, with early adoption permitted. The Company is currently evaluating ASU 2016-02 and its impact on its consolidated financial statements. | ||
| In March 2016, the FASB issued Accounting Standards Update No. 2016-09, Compensation-Stock Compensation (Topic 718): Improvements to Employee Share-Based Payment Accounting (“ASU 2016-09”), which simplifies certain provisions associated with the accounting for stock compensation. Among other things, ASU 2016-09 requires companies to record excess tax benefits and tax deficiencies as income tax benefit or expense in the statement of income and eliminates the requirement to reclassify cash flows related to excess tax benefits from operating activities to financing activities in the statement of cash flows. ASU 2016-09 is effective for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2016, with early adoption permitted. The Company is currently reviewing and evaluating this guidance and its impact on its consolidated financial statements. | ||
| In June 2016, the FASB issued Accounting Standards Update No. 2016-13, Financial Instruments – Credit Losses – Measurement of Credit Losses on Financial Instruments, which introduces a model based on expected losses to estimate credit losses for most financial assets and certain other instruments. In addition, for available-for-sale debt securities with unrealized losses, the losses will be recognized as allowances rather than reductions in the amortized cost of the securities. The standard is effective for annual reporting periods beginning after December 15, 2019, with early adoption permitted for annual reporting periods beginning after December 15, 2018. The Company is evaluating the impact of the adoption on our consolidated balance sheet, results of operations, cash flows and disclosures. |
| NOTE 3 - | OTHER CURRENT ASSETS |
| As of December 31, | ||||||||
| 2016 | 2015 | |||||||
| Government institutions | $ | 41 | $ | 102 | ||||
| Grant receivable from Israeli Innovation Authority | - | 53 | ||||||
| Advance to suppliers | 222 | - | ||||||
| Other | - | 25 | ||||||
| $ | 263 | $ | 180 | |||||
| F-28 |
MOTUS GI HOLDINGS, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
Expressed in U.S. dollars in thousands
| NOTE 4 - | INVENTORY |
| As of December 31, | ||||||||
| 2016 | 2015 | |||||||
| Components | $ | 81 | - | |||||
| $ | 81 | - | ||||||
| NOTE 5 - | FIXED ASSETS, NET |
| Computer and software | Leasehold improvements | Laboratory equipment | Total | |||||||||||||
| Cost: | ||||||||||||||||
| Balance - January 1, 2016 | $ | 106 | $ | 75 | $ | 120 | $ | 301 | ||||||||
| Additions | 6 | 8 | 16 | 30 | ||||||||||||
| Balance - December 31, 2016 | 112 | 83 | 136 | 331 | ||||||||||||
| Accumulated depreciation: | ||||||||||||||||
| Balance - January 1, 2016 | 84 | 13 | 47 | 144 | ||||||||||||
| Additions | 21 | 8 | 17 | 46 | ||||||||||||
| Balance - December 31, 2016 | 105 | 21 | 64 | 190 | ||||||||||||
| Net book value: | ||||||||||||||||
| December 31, 2016 | 7 | 62 | 72 | 141 | ||||||||||||
| December 31, 2015 | $ | 22 | $ | 62 | $ | 73 | $ | 157 | ||||||||
| F-29 |
MOTUS GI HOLDINGS, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
Expressed in U.S. dollars in thousands
| NOTE 6 - | OTHER CURRENT LIABILITIES |
| As of December 31, | ||||||||
| 2016 | 2015 | |||||||
| Wage-related liabilities (1) | $ | 342 | $ | 162 | ||||
| Accrued expenses | 224 | 41 | ||||||
| Taxes payable | 79 | 29 | ||||||
| Other | - | 4 | ||||||
| $ | 645 | $ | 236 | |||||
| (1) Includes accrued vacation and convalescence pay | $ | 113 | $ | 57 | ||||
| NOTE 7 - | COMMITMENTS AND CONTINGENCIES |
| A. | Motus Ltd. received approval from the Israel Innovation Authority (previously the Office of the Chief Scientist) to participate in certain R&D programs from 2011 until 2016 within the framework of determined budgets and time periods. As of December 31, 2016, Motus Ltd. had received an accumulated amount of $ 1,378,000 (“the Grant”). | |
| According to the agreement with the Israel Innovation Authority, the Company will pay royalties of 3% of sales up to an amount equal to the accumulated grant received. Repayment of the grant is contingent upon the successful completion of the Company’s R&D programs and generating sales. The Company has no obligation to repay these grants, if the R&D program fails, is unsuccessful or aborted or if no sales are generated. The Company had not yet generated sales as of December 31, 2016; therefore, no liability was recorded in these consolidated financial statements. | ||
| B. | Motus Ltd. entered into a lease agreement for its facilities on January 1, 2015. According to the lease agreement, Motus Ltd. will pay monthly rent of approximately $7,000. This agreement is for a period of 5 years ending on December 31, 2019, and Motus Ltd. has an option to renew the agreement for an additional 3-year period. | |
| C. | The Company has a severance liability to its CEO and COO of approximately $ 400,000 in the event that they are terminated or leave due to good cause, as outlined in their employee agreements. Management estimates that the likelihood of payment is remote; therefore, no liability was reflected in these consolidated financial statements. |
| F-30 |
MOTUS GI HOLDINGS, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
| NOTE 8 - | SHARE CAPITAL |
| Formation shares | |
| During October and November 2016, the Company issued 1,650,000 common shares pursuant to the formation of the Company. | |
| Shares exchange | |
| As detailed in Note 1, during the Recapitalization Transaction the Company issued 4,000,000 common shares in exchange for 100% of the issued and outstanding and preferred shares of Motus Ltd. | |
Registration Rights
In connection with the 2017 Private Placement, the Company entered into a registration rights agreement (the “Registration Rights Agreement”) with the 2017 Private Placement investors, (the “Investors”). The Company is required to file with the SEC after the date of the final closing of the 2017 Private Placement (the “Registration Filing Date”), a registration statement covering the resale of the shares of our common stock held by the Investors (the “Investor Shares”) issued in the 2017 Private Placement, as well as the shares of our common stock underlying the Series A Convertible Preferred Stock issued in the 2017 Private Placement (together with the Investor Shares, the “Registrable Securities”). The Company is also required to use commercially reasonable efforts to have the registration statement declared effective within one hundred and fifty (150) days after the registration statement is filed (the “Effectiveness Deadline”); provided however, that if the Company signs a letter of intent or comparable agreement with an underwriter which contemplates an Initial Public Offering (“IPO”) or holds an organizational meeting for an IPO, or otherwise orally engages an underwriter to begin working with the Company towards an IPO prior to the Effectiveness Deadline (the “IPO Process Commencement Date”), then the Company shall file a joint registration statement covering the primary shares to be issued in the IPO and the resale of the Registrable Securities, and in such event the Registration Filing Date shall be extended to a date that is seventy five (75) calendar days after the IPO Process Commencement Date and the Effectiveness Deadline shall be extended to a date that is one hundred twenty (120) calendar days after the initial filing of the Registration Statement with the Commission. If the IPO is abandoned at any time, then the Registration Filing Date will be sixty (60) calendar days from the actual date of abandonment and the Effectiveness Deadline will be one hundred and fifty (150) calendar days after the date of abandonment. The Company is also required to keep the registration statement continuously effective under the Securities Act for a period of one year or for such shorter period ending on the earlier to occur of the date when all the Registrable Securities covered by the registration statement have been sold or such time as all of the Registrable Securities covered by the registration statement can be sold under Rule 144 without any volume limitations.
If this registration statement is not declared effective on or before the Effectiveness Deadline, the Company will be required to pay to each holder of Registrable Securities purchased in the 2017 Private Placement an amount in cash equal to one-half of one percent (0.5%) of such holder’s investment amount on every thirty (30) day anniversary of such Effectiveness Deadline until such failure is cured. The payment amount shall be prorated for partial thirty (30) day periods. The maximum aggregate amount of payments to be made by us as the result of such failure, shall be an amount equal to six percent (6%) of each holder’s investment amount. Notwithstanding the foregoing, no payments shall be owed with respect to any period during which all of the holder’s Registrable Securities may be sold by such holder without restriction under Rule 144.
The Company shall keep the registration statement “evergreen” for one (1) year from the date it is declared effective by the SEC or until Rule 144 of the Securities Act is available to the holders of Registrable Securities purchased in the 2017 Private Placement with respect to all of their shares, whichever is earlier. | |
| Private placement | |
| On December 22, 2016, prior to the consummation of the Recapitalization Transaction, the Company consummated a private placement transaction as part of the Recapitalization Transaction. The private placement consisted of units each consisting of three-quarter of a share of common stock, par value $0.0001 and one-quarter a share of convertible preferred series A stock, par value $0.0001. | |
| Pursuant to the private placement, on December 22, 2016, the Company issued 1,211,655 common shares and 403,885 preferred shares for total consideration of $8,077,000. | |
| Each share of preferred series A stock is initially convertible at the option of the holder into one share of common stock. Each share of series A preferred stock will automatically convert into common stock at the earliest to occur of (a) three years from the initial closing of the private placement or (b) notice by the Company to the holders of series A preferred stock that the Company has elected to convert all outstanding shares (“mandatory conversion date”). Holders of the series A preferred stock will not be entitled to receive dividends. The preferred stockholders will vote on an as-converted basis with the common stockholders, and upon any liquidation, dissolution or winding-up of the Company, the holders of the series A preferred stock will be entitled to receive, for each share of preferred stock an amount equal to the stated value plus any accrued and unpaid dividends thereon before any distribution or payment may be made to the common stockholders. | |
| The series A preferred stock includes the right, as a group, to receive: (i) a percentage of the net sales of the Pure-Vu™ medical device system, and (ii) a percentage of the proceeds, if any, received by the Company in connection with the licensing of the Pure-Vu™ system (“Royalty Payment Rights”). For additional information, see “Royalty payment rights on series A preferred stock” on the following page. | |
| Exchange of convertible notes | |
| On December 22, 2016, Motus Ltd. held convertible notes in the amount of $14,596,683, inclusive of accrued interest. In connection with the Recapitalization Transaction, the Company exchanged the convertible notes for approximately 2,432,808 shares of common stock and 810,960 shares of series A preferred stock. |
| F-31 |
MOTUS GI HOLDINGS, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
| NOTE 8 - | SHARE CAPITAL (Cont.) |
| Convertible notes warrants | |
| As a result of the Recapitalization Transaction, the Company issued 907,237 convertible note warrants (the “CNA Warrants”) to replace the warrants previously issued to the convertible note holders. The five-year warrants are exercisable for the Company’s common stock at an exercise price of $5.00 per share. | |
| Royalty payment rights on series A preferred stock | |
| The Royalty Payment Rights entitle the holders in aggregate, to a royalty in an amount of: |
| ● | 3% of net sales subject to a maximum in any calendar year equal to the total dollar amount of units closed on in the private placement offering; and | |
| ● | 5% of licensing proceeds subject to a maximum in any calendar year equal to the total dollar amount of units closed on in the private placement. |
| Even if converted pursuant to the mandatory conversion as defined above, the royalty right certificate will remain in the hands of the holder in addition to the common stock to be issued following conversion. | |
| If converted at the option of the holder prior to the mandatory conversion, the holder will lose all rights to future royalty payments. | |
| The royalty rights certificate was recorded as a liability at fair value in the consolidated financial statements at December 31, 2016. The Company recognized a liability at fair value as “other long-term liabilities” with regard to the Royalty Payment Rights in an amount of $1,282,000. The fair value adjustment from the date of inception on December 22, 2016 until December 31, 2016 was immaterial. | |
| In addition, the Company issued a royalty rights certificate to the agent with substantially similar terms as those of the Series A Convertible Preferred Stock, which grants the agent the right, in the aggregate, to receive 10% of the amount of payments paid to the holders of the Series A Convertible Preferred Stock. | |
| The Company measured the fair value according to the discounted cash flow method. The fair value was allocated to the royalty rights and the residual value was allocated to the preferred shares and recorded as equity. The following assumptions (level 3 measurements) were used: |
| Year ended December 31, | ||||||||
| 2016 | 2015 | |||||||
| Discount rate | 20 | % | - | |||||
| In accordance with ASC-820-10-50-2(g), the Company performed a sensitivity analysis of the liability, which was classified as a level 3 financial instrument. The Company recalculated the fair value of the liability by applying a +/- 2% change to the input variable in the discounted cash flow model, namely, the discount rate. A 2% decrease in the discount rate would increase the liability by approximately $150,000; a 2% increase in the discount rate would decrease the liability by approximately $131,000. |
| F-32 |
MOTUS GI HOLDINGS, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
| NOTE 9 - | SHARE-BASED COMPENSATION (Cont.) |
| Employee stock option grant | |
| The Company has one valid option plan that was approved in 2009. This plan was adjusted in 2016 following the reverse recapitalization transaction on December 22, 2016. | |
| According to the original plan, employees and service providers were entitled to receive options to purchase common shares of Motus Ltd. Following the recapitalization, the options granted will entitle their holder to purchase common shares of the Company. As a result, the number of options and exercise price per share were adjusted in a technical manner such that there was no change in the fair value of the awards under the adjusted plan. All number of options and exercise prices in this Note have been restated to reflect the adjusted option plan. | |
| The following table presents the number of options outstanding according to the terms of the adjusted plan compared to the original plan: |
| As of December 31, 2016 | ||||||||
| Original Plan | Adjusted Plan | |||||||
| Total options outstanding | 1,757,730 | 125,730 | ||||||
| From 2012 through 2015, the Company granted its employees, not including its CEO, options to purchase an aggregate of 90,108 shares of common stock of the Company at an exercise price ranging from $2.38 to $2.52 per share. The options will expire 10 years from the date of issuance. Some of the options have a vesting period of 3 years, while others are upon the achievement of certain milestones. The remaining unvested shares will vest upon 1) the Company’s obtainment of CE approval of its system; and 2) upon the enrollment of the first patient in a post market study with a “prep-less” indication, or the sale of the first 1,000 disposables. | |
| On April 2, 2014, the Company granted its CEO options to purchase 67,238 shares of the Company’s common stock. Of the total options granted, 48,527 options will vest upon the achievement of certain milestones, as detailed, above and additional milestones including the gross return in multiples on preferred A shares. The remaining 18,711 will vest over a period of 3 years. The exercise price of the options are $2.38 per share. | |
| A summary of the Company’s option activity related to options to employees and related information is as follows: |
| Shares | Weighted Average Exercise Price | Weighted average remaining contractual term (years) | ||||||||||
| Options outstanding, December 31, 2014 | 123,175 | $ | 2.38 | 9.7 | ||||||||
| Options granted | 34,171 | 2.52 | ||||||||||
| Options forfeited or expired | (38,053 | ) | 2.38 | |||||||||
| Options outstanding, December 31, 2015 | 119,293 | 2.42 | 9 | |||||||||
| Options forfeited or expired | (8,582 | ) | 2.38 | |||||||||
| Options outstanding, December 31, 2016 | 110,711 | $ | 2.42 | 8 |
| F-33 |
MOTUS GI HOLDINGS, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
| NOTE 9 - | SHARE-BASED COMPENSATION (Cont.) |
| The number of options that had vested as of December 31, 2016 and December 31, 2015 was 67,908 and 35,524, respectively. | |
| The aggregate intrinsic value (the difference between the fair market value of the Company’s common shares on December 31, 2016 and December 31, 2015, respectively and the exercise price, multiplied by the number of in-the-money options on those dates) was $175,203 and $0 as of December 31, 2016 and December 31, 2015, respectively. | |
| Compensation expense recorded by the Company in respect of options granted in accordance with ASC 718-10 for the years ended December 31, 2016 and 2015 amounted to $20 thousand and $11 thousand, respectively. | |
| The fair value of the options is estimated at the date of grant using Black-Scholes options pricing model with the following assumptions (level 3 measurement) used in the calculation: |
| Year ended December 31, | ||||||||
| 2016 | 2015 | |||||||
| Expected volatility | 60 | % | 60 | % | ||||
| Risk-free interest | 1.5 | % | 1.5 | % | ||||
| Dividend yield | 0 | % | 0 | % | ||||
| Expected life (in years) | 5 | 5 | ||||||
| Options to service providers | |
| The Company accounts for option to purchase common shares issued to non-employees using the guidance of ASC 505-50, “Equity-Based Payments to Non-Employees”, whereby the fair value of such options is determined at the earlier of the date at which the non-employee’s performance is completed or a performance commitment is reached. | |
| In 2012 and 2014, the Company granted 7,510 and 7,509 options to its service providers, respectively, to purchase the Company’s common stock at an exercise price of $2.38 per share. The options vest over a period of 3 years and will expire 10 years from the date of issuance. |
| As of December 31, | ||||||||
| 2016 | 2015 | |||||||
| Options outstanding | 15,019 | 15,019 | ||||||
| Options vested | 11,392 | 7,490 | ||||||
| Share-based compensation expense recorded by the Company with regard to options to service providers was $34 thousand and $0, as of December 31, 2016 and December 31, 2015, respectively. |
| F-34 |
MOTUS GI HOLDINGS, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
Expressed in U.S. dollars in thousands
| NOTE 10 - | CONVERTIBLE NOTES |
| On June 9, 2015, Motus Ltd. signed a convertible note agreement with a number of lenders according to which the Company received approximately $4.1 million. During 2016, Motus Ltd. signed three additional amendments to the original agreement to raise an additional amount of approximately $9.6 million. The convertible notes accrued annual interest of 10%. In addition, each lender received options to purchase ordinary shares of Motus Ltd. in an amount equal to 33% of the amount received. As a part of the Recapitalization Transaction, the convertible notes were converted into common shares of the Company. See Note 8 for details regarding this transaction. | |
| The Company concluded the value of the convertible notes were predominantly based on a fixed monetary amount known at the date of issuance as represented by the 10% discount on the Motus Ltd.’s common shares to be sold in a qualified financing round (as defined in Motus Ltd.’s Articles of Association). Accordingly, the convertible note was classified as debt and was measured at its fair value, pursuant to the provisions of ASC 480-10, “Accounting for Certain Financial Instruments with Characteristics of Both Liabilities and Equity”. The fair value of the convertible note was measured based on observable inputs as the fixed monetary value of the variable number of shares to be issued upon conversion (level 2 measurement). |
| NOTE 11 - | RESEARCH AND DEVELOPMENT EXPENSES, NET |
| Year ended December 31, | ||||||||
| 2016 | 2015 | |||||||
| Salaries and fringe benefits (*) | $ | 1,059 | $ | 1,258 | ||||
| Subcontractors | 1,473 | 1,026 | ||||||
| Clinical Study | 245 | - | ||||||
| Materials | 143 | 1,044 | ||||||
| Patents | 215 | 92 | ||||||
| Travel | 58 | 65 | ||||||
| Other | 30 | - | ||||||
| Deprecation | 12 | 38 | ||||||
| 3,235 | 3,523 | |||||||
| Less government grants | (156 | ) | (363 | ) | ||||
| $ | 3,079 | $ | 3,160 | |||||
| (*) | Includes share-based compensation expenses in the amount of $24 thousand and $11 thousand for the years ended in December 31, 2016 and December 31, 2015, respectively. |
| F-35 |
MOTUS GI HOLDINGS, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
Expressed in U.S. dollars in thousands
| NOTE 12 - | MARKETING EXPENSES |
| Year ended December 31, | ||||||||
| 2016 | 2015 | |||||||
| Salaries and fringe benefits (*) | $ | 824 | $ | 203 | ||||
| Professional services | 64 | 209 | ||||||
| Travel | 84 | - | ||||||
| Others | 62 | 3 | ||||||
| $ | 1,034 | $ | 415 | |||||
| (*) | Includes share-based compensation expenses in the amount of $6 thousand for the year ended December 31, 2016. |
| NOTE 13 - | GENERAL AND ADMINISTRATIVE EXPENSES |
| Year ended December 31, | ||||||||
| 2016 | 2015 | |||||||
| Salaries and fringe benefits (*) | $ | 705 | $ | 874 | ||||
| Rental fees | 115 | 149 | ||||||
| Professional services | 449 | 405 | ||||||
| Other salaries benefits (**) | 146 | 147 | ||||||
| Office expenses | 129 | 102 | ||||||
| Depreciation | 34 | 7 | ||||||
| Travel | 122 | 3 | ||||||
| Others | 194 | 63 | ||||||
| $ | 1,894 | $ | 1,750 | |||||
| (*) | Includes share based payment expenses in the amount of $24 thousand for the year ended December 31, 2016. | |
| (**) | Includes vehicles maintenance and benefits for all employees. |
| F-36 |
MOTUS GI HOLDINGS, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
Expressed in U.S. dollars in thousands
| NOTE 14 - | FINANCE EXPENSES, NET |
| Year ended December 31, | ||||||||
| 2016 | 2015 | |||||||
| Bank fees and interest | $ | 86 | $ | 5 | ||||
| Change in fair value and interest on convertible notes | 1,907 | 633 | ||||||
| Exchange rate differences | (27 | ) | (1 | ) | ||||
| $ | 1,966 | $ | 637 | |||||
| NOTE 15 - | INCOME TAXES |
| The Company is subject to income taxes under Israeli and U.S. tax laws: | |
| Corporate tax rates | |
| The Company is subject to Israeli corporate income tax rate of 26.5% in 2015, 25% in 2016, 24% in 2017, and 23% from 2018 and years thereafter. | |
| The Company is subject to a blended U.S. income tax rate (federal as well as state corporate tax) of approximately 35%. |
| A. | As of December 31, 2016, the Company generated net operating losses in Israel of approximately $25,628 thousand which may be carried forward and offset against taxable income in the future for an indefinite period. | |
| B. | The Company is still in its development stage and has not yet generated revenues; therefore, it is more likely than not that sufficient taxable income will not be available for the tax losses to be utilized in the future. Therefore, a valuation allowance was recorded to reduce the deferred tax assets to its recoverable amounts. |
| As of December 31, | ||||||||
| 2016 | 2015 | |||||||
| Deferred tax asset: | ||||||||
| Net loss carry-forward | $ | 6,151 | $ | 4,670 | ||||
| Valuation allowance | (6,151 | ) | (4,670 | ) | ||||
| Net deferred tax asset | $ | - | $ | - | ||||
| F-37 |
MOTUS GI HOLDINGS, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
| NOTE 16 - | BASIC AND DILUTED NET LOSS PER COMMON SHARE |
| The basic and diluted net loss per share and weighted average number of common shares used in the calculation of basic and diluted net loss per share are as follows (in thousands, except share and per share data): | |
| As the inclusion of common share equivalents in the calculation would be anti-dilutive for all periods presented, diluted net loss per share is the same as basic net loss per share. | |
| The weighted average number of common shares outstanding has been retroactively restated as of December 31, 2015 to reflect the equivalent number of common shares received by the accounting acquirer as a result of the reverse recapitalization as if these common shares had been outstanding as of the beginning of the earliest period presented. |
| NOTE 17 - | SUBSEQUENT EVENTS |
| A. | On January 30, 2017, the Company completed the second closing of the private placement (see Note 8). The Company raised $2.9 million for 146,865 units consisting of 3/4 common stock and 1/4 series A preferred stock. | |
| B. | On February 24, 2017, the Company completed the third and final closing of the private placement (see Note 8). The Company raised $4.4 million for 219,418 units consisting of 3/4 common stock and 1/4 series A preferred stock. | |
| C. | In connection with the aforementioned private placement, the Company issued 403,632 warrants to purchase 403,632 of the Company’s common stock to the placement agent at an exercise price equal to the fair value of the common stock on the grant date. The warrants are exercisable for a period of 5 years from the grant date and provide for a cashless exercise. |
| F-38 |
Until , 2017 (the 25th day after the commencement of this offering), all dealers that effect transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to the dealers’ obligation to deliver a prospectus when acting as an underwriter and with respect to their unsold allotments or subscriptions.
MOTUS GI HOLDINGS, INC.
Shares
Common Stock
PROSPECTUS
_____ __, 2017
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
| ITEM 13. | OTHER EXPENSES OF ISSUANCE AND DISTRIBUTION |
The following table sets forth all fees and expenses to be paid by us, other than estimated underwriting discounts and commissions, in connection with this offering. All amounts shown are estimates except for the registration fee, the FINRA filing fee and the exchange listing fee.
| SEC Registration Fee | $ | * | |||
| FINRA filing fee | $ | * | |||
| Securities exchange fee | $ | * | |||
| Printing and engraving expenses | $ | * | |||
| Accounting Fees and Expenses | $ | * | |||
| Blue Sky fees and expenses (including legal fees) | $ | * | |||
| Transfer agent and registrar fees and expenses | $ | * | |||
| Legal Fees and Expenses | $ | * | |||
| Miscellaneous Fees and Expenses | $ | * | |||
| Total | $ | * |
* To be filed by amendment.
| ITEM 14. | INDEMNIFICATION OF OFFICERS AND DIRECTORS |
Section 145 of the Delaware General Corporation Law (the “DGCL”) provides, in general, that a corporation incorporated under the laws of the State of Delaware, as we are, may indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action, suit or proceeding (other than a derivative action by or in the right of the corporation) by reason of the fact that such person is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another enterprise, against expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by such person in connection with such action, suit or proceeding if such person acted in good faith and in a manner such person reasonably believed to be in or not opposed to the best interests of the corporation and, with respect to any criminal action or proceeding, had no reasonable cause to believe such person’s conduct was unlawful. In the case of a derivative action, a Delaware corporation may indemnify any such person against expenses (including attorneys’ fees) actually and reasonably incurred by such person in connection with the defense or settlement of such action or suit if such person acted in good faith and in a manner such person reasonably believed to be in or not opposed to the best interests of the corporation, except that no indemnification will be made in respect of any claim, issue or matter as to which such person will have been adjudged to be liable to the corporation unless and only to the extent that the Court of Chancery of the State of Delaware or any other court in which such action was brought determines such person is fairly and reasonably entitled to indemnity for such expenses.
| II-1 |
Our certificate of incorporation and bylaws provide that we will indemnify our directors, officers, employees and agents to the extent and in the manner permitted by the provisions of the DGCL, as amended from time to time, subject to any permissible expansion or limitation of such indemnification, as may be set forth in any amendment by stockholders or directors resolution.
Any repeal or modification of these provisions approved by our stockholders will be prospective only and will not adversely affect any limitation on the liability of any of our directors or officers existing as of the time of such repeal or modification.
We have director and officer liability insurance to cover liabilities our directors and officers may incur in connection with their services to us, including matters arising under the Securities Act.
We have entered into indemnification agreements with certain of our directors and officers whereby we have agreed to indemnify those directors and officers to the fullest extent permitted by law, including indemnification against expenses and liabilities incurred in legal proceedings to which the director or officer was, or is threatened to be made, a party by reason of the fact that such director or officer is or was a director, officer, employee or agent of Motus GI Holdings, Inc. (the “Company”), provided that such director or officer acted in good faith and in a manner that the director or officer reasonably believed to be in, or not opposed to, the best interests of the Company.
The Underwriting Agreement to be filed as Exhibit 1.1 to this registration statement provides for indemnification by the underwriters of us, our executive officers and directors, and by us of the underwriters for certain liabilities, including liabilities arising under the Securities Act and otherwise.
| ITEM 15. | RECENT SALES OF UNREGISTERED SECURITIES |
Since January 1, 2016, the Company made sales of the following unregistered securities:
Original Issuances of Stock, Warrants and Payment Rights Certificates
Formation of Holdings
In connection with our formation in September 2016, we sold an aggregate of 1,650,000 shares of our common stock for an aggregate of $82,000 ($0.05 per share), which includes 450,000 shares of our common stock owned by an affiliate of Aegis Capital Corp., the placement agent (“Placement Agent”) for our private placement, for which closings occurred December 22, 2016 through February 24, 2017 (the “2017 Private Placement”), described below.
2017 Private Placement
From December 2016 through February of 2017, we sold an aggregate of 4,743,311 shares of our common stock and 1,581,128 shares of Series A Convertible Preferred Stock at a price of $5.00 per Unit, inclusive of 2,432,808 shares of our common stock and 810,960 shares of Series A Convertible Preferred Stock issued pursuant to the Exchange of Convertible Notes, to 229 accredited investors.
In connection with the 2017 Private Placement, we issued (i) the Placement Agent Warrants to the Placement Agent to purchase 403,632 shares of our common stock with an exercise price of $5.00 per share and (ii) the Placement Agent Royalty Payment Rights Certificates to receive, in the aggregate, 10% of the amount of payments paid to the holders of the Series A Convertible Preferred Stock.
| II-2 |
Share Exchange Transaction
On December 1, 2016 Motus GI Medical Technologies Ltd. (“Opco”), and the holders of all issued and outstanding shares of capital stock of Opco (the “Opco Stockholders”), entered into a share exchange agreement (the “Share Exchange Agreement”) with us. Pursuant to the terms of the Share Exchange Agreement, as a condition of and contemporaneously with the initial closing (the “Initial Closing”) of the 2017 Private Placement, the Opco Stockholders sold to us, and we acquired, all of the issued and outstanding shares of capital stock of Opco (the “Share Exchange Transaction”) and Opco became our wholly-owned subsidiary. Pursuant to the Share Exchange Transaction (i) the Opco Stockholders received an aggregate of 4,000,000 shares of our common stock in exchange for all of the issued and outstanding shares of capital stock of Opco, (ii) the Convertible Notes (as defined below) and the Convertible Note Warrants (as defined below) were exchanged for our securities, as described below, and (iii) all of the issued and outstanding options to purchase or otherwise acquire shares of Opco capital stock that were outstanding and unexercised as of immediately prior to the Initial Closing were substituted for 125,730 options to acquire shares of our common stock pursuant to our 2016 Equity Incentive Plan.
Exchange of Convertible Notes
Pursuant to the terms of a convertible note agreement (the “CNA”), as amended, Opco issued convertible notes (the “Convertible Notes”) in a series of closings from June 2015 through November 2016, in an aggregate amount of $14,596,683 (inclusive of accrued interest through December 22, 2016, the date of the Initial Closing). Certain related parties purchased Convertible Notes pursuant to the CNA, see “Certain Relationships and Related Party Transactions - Convertible Note, Convertible Warrant, and 2017 Private Placement - Related Party Participation.” At the Initial Closing, the holders of the Convertible Notes (“Convertible Holders”) exchanged their Convertible Notes (the “Exchange of Convertible Notes”), together with accrued and unpaid interest thereon calculated through the date of the Initial Closing at a rate of 10% per annum, for Units of the 2017 Private Placement, at a price of $4.50 per Unit. As a result, upon consummation of the Initial Closing, the Convertible Notes were exchanged for Units representing (i) 2,432,808 shares of our common stock (inclusive of shares of our common stock resulting from the accrued and unpaid interest of the Convertible Notes through the date of the Initial Closing) and (ii) 810,960 shares of Series A Convertible Preferred Stock (inclusive of shares of Series A Convertible Preferred Stock resulting from the accrued and unpaid interest of the Convertible Notes through the date of the Initial Closing).
Exchange of Convertible Note Warrants
In connection with, and pursuant to the terms of, the CNA, each Convertible Holder also received a seven (7) year warrant (the “Convertible Note Warrants”) to purchase Preferred A Shares of Opco, nominal value in Israeli New Shekel (“NIS”) 0.01 per share, with an exercise price per share of $1.00 (the “Convertible Note Warrant Exercise Price”). Each Convertible Note Warrant entitled the holder to purchase that number of shares of Preferred A Shares of Opco equal to thirty-three percent (33%) of the principal amount of the Convertible Note in connection with which it was issued. Convertible Note Warrants to purchase an aggregate 4,536,186 shares of Preferred A Shares of Opco with an exercise price of $1.00 were issued in connection with the CNA. Certain related parties held Convertible Note Warrants pursuant to the CNA, see “Certain Relationships and Related Party Transactions - Convertible Note, Convertible Warrant, and 2017 Private Placement - Related Party Participation.” At the Initial Closing, the holders of the Convertible Note Warrants exchanged their Convertible Note Warrants for five (5) year warrants (the “Exchange Warrants”) to purchase an aggregate 907,237 shares of our common stock at an exercise price of $5.00 per share, such amount being equal to thirty-three percent (33%) of the principal amount of the Convertible Notes divided by $5.00.
Service Provider Stock and Warrants
In May 2017, we issued a service provider (a) 90,000 shares of our common stock, subject to a lock-up agreement, and (b) five (5) year warrants to purchase 30,000 shares of our common stock with an exercise price of $8.00 per share, as partial payment for services pursuant to a consulting agreement between the service provider and us.
During August and September of 2017, we issued a service provider an aggregate of 2,778 shares, and pursuant to the terms of the agreement with such service provider we are obligated to issue one additional installment of 1,389 shares to such service provider in October 2017.
Issuance Pursuant to Exercise of Stock Option
In May 2017, we issued 754 shares of our common stock pursuant to the exercise of a stock option.
| II-3 |
Stock Options
Since January 1, 2016, we have granted stock options under our 2016 Equity Incentive Plan to purchase an aggregate of 2,099,499 shares of our common stock, at exercise prices ranging from $2.38 to $4.50 per share, following the modification of share-based payment awards on September 29, 2017. Pursuant to the terms of the 2016 Equity Incentive Plan, as of September 30, 2017, 262,654 of the shares covered by such grants have been forfeited, cancelled, returned to the us for failure to satisfy vesting requirements or otherwise terminated without payment, and such shares are no longer counted against the maximum share limitation of the 2016 Equity Incentive Plan.
Unrestricted Stock Awards
Since January 1, 2016, we have granted unrestricted stock awards under our 2016 Equity Incentive Plan for an aggregate of 5,000 shares of our common stock.
Securities Act Exemptions
We deemed the offers, sales and issuances of the securities described above under “Original Issuances of Stock, Warrants and Payment Rights Certificates” to be exempt from registration under the Securities Act of 1933, as amended (the “Securities Act”), in reliance on Section 4(a)(2) of the Securities Act, including Regulation D and Rule 506 promulgated thereunder, relative to transactions by an issuer not involving a public offering. All purchasers of securities in transactions exempt from registration pursuant to Regulation D represented to us that they were accredited investors and were acquiring the shares for investment purposes only and not with a view to, or for sale in connection with, any distribution thereof and that they could bear the risks of the investment and could hold the securities for an indefinite period of time. The purchasers received written disclosures that the securities had not been registered under the Securities Act and that any resale must be made pursuant to a registration statement or an available exemption from such registration.
We deemed the grants of stock options and issuances of our common stock upon exercise of such options, and the restricted share awards, described above under “Stock Options” to be exempt from registration under the Securities Act in reliance on Section 4(a)(2) of the Securities Act, including Regulation D and Rule 506 promulgated thereunder and Rule 701 of the Securities Act as offers and sales of securities under compensatory benefit plans and contracts relating to compensation in compliance with Rule 701. Each of the recipients of securities in any transaction exempt from registration either received or had adequate access, through employment, business or other relationships, to information about us.
All certificates representing the securities issued in the transactions described in this Item 15 included appropriate legends setting forth that the securities had not been offered or sold pursuant to a registration statement and describing the applicable restrictions on transfer of the securities. There were no underwriters employed in connection with any of the transactions set forth in this Item 15. All recipients had adequate access, through their relationships with us, to information about us. The sales of these securities were made without any general solicitation or advertising.
| II-4 |
| ITEM 16. | EXHIBITS AND FINANCIAL STATEMENT SCHEDULES |
| II-5 |
| 10.11 | Form of Israeli Option Grant to Israeli Non-Employees and Controlling Shareholders Agreement | |
| 10.12† | Employment Agreement, dated December 22, 2016, between the Company and Mark Pomeranz | |
| 10.13 | Lease, dated April 13, 2017, between Company and Victoriana Building, LLC | |
| 10.14 | Form of Subscription Agreement for Convertible Notes Offering | |
| 10.15 | Finders Agreement, dated October 14, 2016, between the Company and Aegis Capital Corporation | |
| 10.16 | Finders Agreement, dated December 22, 2016, between the Company and Aegis Capital Corporation | |
| 10.17† | Form of Indemnification Agreement | |
| 10.18† | Employment Agreement, dated August 16, 2017, between the Company and Andrew Taylor | |
| 21.1 | List of Subsidiaries of the Company | |
| 23.1* | Consent of Brightman Almagor Zohar & Co. | |
| 23.2 * | Consent of Lowenstein Sandler LLP (included in Exhibit 5.1) | |
| 24.1 | Power of Attorney (included on the signature page) |
| * | To be filed by amendment. | |
| + | As permitted by Item 601(b)(2) of Regulation S-K, certain schedules to this agreement have not been filed herewith. The company will furnish supplementally a copy of any omitted schedule to the SEC upon request. | |
| † | Indicates management contract or compensatory plan. |
| II-6 |
| ITEM 17. | UNDERTAKINGS |
(a) The undersigned Registrant hereby undertakes to provide to the underwriters at the closing specified in the underwriting agreement certificates in such denominations and registered in such names as required by the underwriters to permit prompt delivery to each purchaser.
(b) Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the Registrant pursuant to the foregoing provisions, or otherwise, the Registrant has been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the Registrant of expenses incurred or paid by a director, officer or controlling person of the Registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the Registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue.
(c) The undersigned Registrant hereby undertakes that:
(1) For purposes of determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the Registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective.
(2) For the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
| II-7 |
Pursuant to the requirements of the Securities Act of 1933, as amended, the registrant has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Ft. Lauderdale, State of Florida on , 2017.
| MOTUS GI HOLDINGS, INC. | ||
| By: | ||
| Name: | Mark Pomeranz | |
| Title: | Chief Executive Officer | |
KNOW ALL MEN BY THESE PRESENTS, that the undersigned officers and directors Motus GI Holdings, Inc., a Delaware corporation (the “Company”), do hereby constitute and appoint each of Mark Pomeranz and Andrew Taylor as his or her true and lawful attorney-in-fact and agent, with full power of substitution and re-substitution, for him and in his name, place, and stead, in any and all capacities, to sign any and all amendments (including post-effective amendments, exhibits thereto and other documents in connection therewith) to this Registration Statement and any subsequent registration statement filed by the registrant pursuant to Rule 462(b) of the Securities Act of 1933, as amended, which relates to this Registration Statement, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorney-in-fact and agent full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection therewith, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorney-in-fact and agent, or his substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, as amended, this registration statement has been signed below by the following persons in the capacities and on the dates indicated.
| Person | Capacity | Date | ||
Chief Executive Officer and Director |
||||
| Mark Pomeranz | (Principal Executive Officer) | , 2017 | ||
Chief Financial Officer |
||||
| Andrew Taylor | (Principal Financial and Accounting Officer) | , 2017 | ||
| David Hochman | Chairman of the Board | , 2017 | ||
| Darren Sherman | Director | , 2017 | ||
| Gary Jacobs | Director | , 2017 | ||
| Samuel Nussbaum | Director | , 2017 | ||
| Shervin Korangy | Director | , 2017 |
| II-8 |